Assay Limits For Medicinal Products . Objective (1.1) this document is intended to provide guidance regarding good manufacturing. This guideline may also be applicable to other types of products, 43. Desired product (1) the protein which has the expected structure, or. (2) the protein which is. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Given pharmaceutical substance or fpp. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Specifications that limit market access by, for example, favouring one manufacturer to the.
from www.pharmamanufacturing.com
And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. This guideline may also be applicable to other types of products, 43. (2) the protein which is. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Desired product (1) the protein which has the expected structure, or. Given pharmaceutical substance or fpp. Specifications that limit market access by, for example, favouring one manufacturer to the. Specification, finished product, chemical, control, test, acceptance criteria, limit, release.
Pharmaceutical USP Regulations USP Updates and for Microbial Testing
Assay Limits For Medicinal Products (2) the protein which is. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. This guideline may also be applicable to other types of products, 43. Given pharmaceutical substance or fpp. Specifications that limit market access by, for example, favouring one manufacturer to the. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. (2) the protein which is. Desired product (1) the protein which has the expected structure, or. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation').
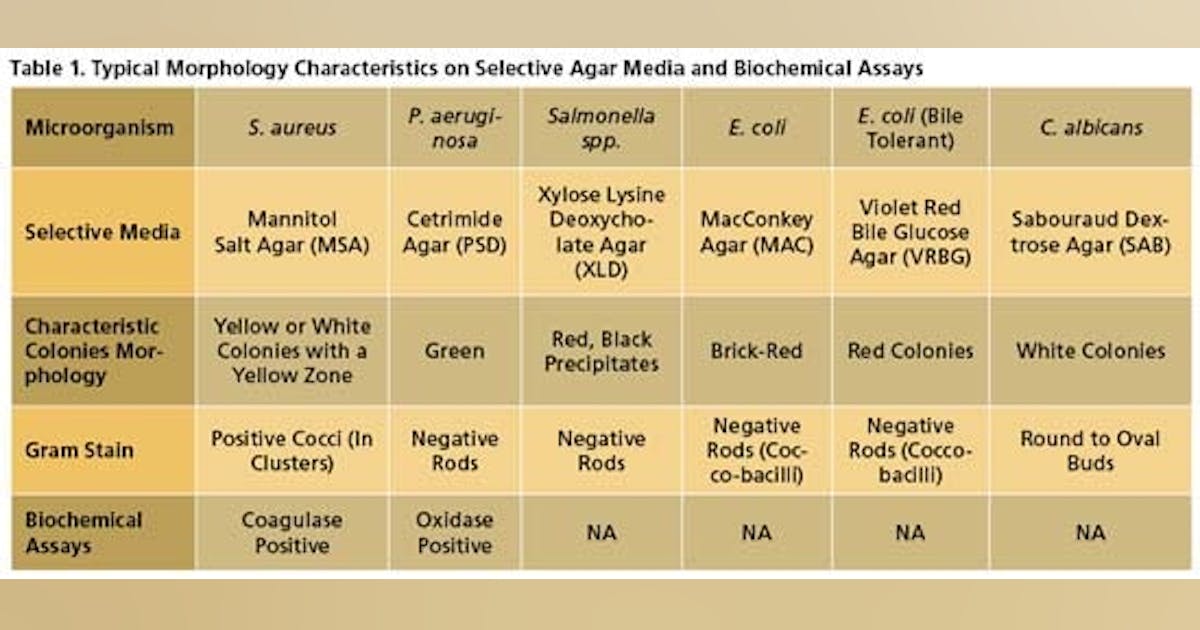
From microbe-investigations.com
USP 62 Microbiological Testing of NonSterile Products Assay Limits For Medicinal Products (2) the protein which is. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Desired product (1) the protein which has the expected structure, or. Given pharmaceutical substance or fpp. Objective (1.1) this. Assay Limits For Medicinal Products.
From www.slideserve.com
PPT 1. Overview of Pharmaceutical Product Development PowerPoint Assay Limits For Medicinal Products Specifications that limit market access by, for example, favouring one manufacturer to the. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Given pharmaceutical substance or fpp. This guideline may also be applicable. Assay Limits For Medicinal Products.
From www.honeymanlaboratories.com
Water Highly Purified, Detecting Endotoxins with TVC and LAL Assay Assay Limits For Medicinal Products Objective (1.1) this document is intended to provide guidance regarding good manufacturing. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Specification, finished product, chemical, control, test, acceptance criteria, limit, release. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Given pharmaceutical. Assay Limits For Medicinal Products.
From www.researchgate.net
[PDF] Assay of active pharmaceutical ingredients in drug products based Assay Limits For Medicinal Products Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). This guideline may also be applicable to other types of products, 43. Desired product (1) the protein which has the expected structure, or. Given pharmaceutical substance or fpp. Objective (1.1) this document is intended to provide. Assay Limits For Medicinal Products.
From www.semanticscholar.org
[PDF] The challenges of potency assay development for cellbased Assay Limits For Medicinal Products And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. (2) the protein which is. This guideline may also be applicable to other types of products, 43. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Desired product (1) the protein which has the expected structure, or.. Assay Limits For Medicinal Products.
From www.researchgate.net
Differences between sterile drugs and nonsterile drugs [8]. Download Assay Limits For Medicinal Products Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Specifications that limit market access by, for example, favouring one manufacturer to the. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). (2). Assay Limits For Medicinal Products.
From www.youtube.com
TABLET CAPSULE WEIGHT VARIATION LIMIT YouTube Assay Limits For Medicinal Products Desired product (1) the protein which has the expected structure, or. Given pharmaceutical substance or fpp. This guideline may also be applicable to other types of products, 43. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). (2) the protein which is. And/or office of communication, outreach and development center for biologics evaluation and research. Assay Limits For Medicinal Products.
From www.presentationeze.com
Cleanroom Classification Information & current Best Assay Limits For Medicinal Products This guideline may also be applicable to other types of products, 43. Desired product (1) the protein which has the expected structure, or. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Objective (1.1) this document is. Assay Limits For Medicinal Products.
From www.youtube.com
Do you know the procedure of Microbial Limit Test? Microbial Analysis Assay Limits For Medicinal Products (2) the protein which is. Specifications that limit market access by, for example, favouring one manufacturer to the. Given pharmaceutical substance or fpp. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. This guideline may also be. Assay Limits For Medicinal Products.
From microbe-investigations.com
USP 71 Sterility Testing Services Microbe Investigations Assay Limits For Medicinal Products (2) the protein which is. Given pharmaceutical substance or fpp. Desired product (1) the protein which has the expected structure, or. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Specifications that limit market access by, for example, favouring one manufacturer to the. And/or office of communication, outreach and development center for biologics evaluation and. Assay Limits For Medicinal Products.
From pediaa.com
What is the Difference Between Limit Test and Assay Assay Limits For Medicinal Products And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. Specifications that limit market access by, for example, favouring one manufacturer to the. Desired product (1) the protein which has the expected structure, or. This guideline. Assay Limits For Medicinal Products.
From www.researchgate.net
Existing microbiological limits and guidelines for Vibrio... Download Assay Limits For Medicinal Products (2) the protein which is. Desired product (1) the protein which has the expected structure, or. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. Specifications that limit market access by, for example, favouring one manufacturer to the. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new. Assay Limits For Medicinal Products.
From www.youtube.com
Assay Limit test Assay Vs Limit test Pharmaceutical Analysis Assay Limits For Medicinal Products Given pharmaceutical substance or fpp. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Specifications that limit market access by, for example, favouring one manufacturer to the. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903. Assay Limits For Medicinal Products.
From www.pharmaqualification.com
Microbial Limit Test for Pharmaceutical Products (PDF included ) Assay Limits For Medicinal Products Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Given pharmaceutical substance or fpp. (2) the protein which is. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Objective (1.1) this document. Assay Limits For Medicinal Products.
From www.differencebetween.net
Difference Between Bioburden and Microbial Limit Test Difference Between Assay Limits For Medicinal Products Specifications that limit market access by, for example, favouring one manufacturer to the. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. This guideline may. Assay Limits For Medicinal Products.
From www.youtube.com
Difference Between BIOBURDEN TEST AND MICROBIAL LIMIT TEST YouTube Assay Limits For Medicinal Products Objective (1.1) this document is intended to provide guidance regarding good manufacturing. (2) the protein which is. Specifications that limit market access by, for example, favouring one manufacturer to the. Given pharmaceutical substance or fpp. This guideline may also be applicable to other types of products, 43. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and. Assay Limits For Medicinal Products.
From www.researchgate.net
Total microbial count and isolated from the collected Assay Limits For Medicinal Products Desired product (1) the protein which has the expected structure, or. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Given pharmaceutical substance or fpp. Specifications that limit market access by, for example, favouring one manufacturer to the. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. (2) the protein which is.. Assay Limits For Medicinal Products.
From ethidelabs.com
Ethide Laboratories Bacterial Endotoxin Limits And Calculations For Assay Limits For Medicinal Products Specification, finished product, chemical, control, test, acceptance criteria, limit, release. This guideline may also be applicable to other types of products, 43. Desired product (1) the protein which has the expected structure, or. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Given pharmaceutical substance or fpp. (2) the protein which is. Specifications that limit. Assay Limits For Medicinal Products.
From www.youtube.com
LIMIT TEST OF ARSENIC FROM PHARMACEUTICAL ANALYSIS, LIMIT TEST, ARSENIC Assay Limits For Medicinal Products Objective (1.1) this document is intended to provide guidance regarding good manufacturing. This guideline may also be applicable to other types of products, 43. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Specification, finished product, chemical, control, test, acceptance criteria, limit, release. (2) the protein which is. Given pharmaceutical substance or fpp. And/or office. Assay Limits For Medicinal Products.
From www.pharmamanufacturing.com
Pharmaceutical USP Regulations USP Updates and for Microbial Testing Assay Limits For Medicinal Products Given pharmaceutical substance or fpp. (2) the protein which is. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. This guideline may also be applicable to other types of products, 43. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Specifications that limit market. Assay Limits For Medicinal Products.
From www.researchgate.net
microbial limits for botanical ingredients. Download Table Assay Limits For Medicinal Products Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Specifications that limit market access by, for example, favouring one manufacturer to the. This guideline may also be applicable to other types of products, 43. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave.,. Assay Limits For Medicinal Products.
From patientparadise.com
What is the future of LAL assays in endotoxin testing? Patientparadise Assay Limits For Medicinal Products Specifications that limit market access by, for example, favouring one manufacturer to the. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. This guideline may also be applicable to other types of products, 43. Given pharmaceutical substance or fpp. (2) the protein which is. And/or office of communication, outreach and development center for biologics evaluation and research. Assay Limits For Medicinal Products.
From pacificbiolabs.com
Microbial Limits Test Pacific BioLabs Assay Limits For Medicinal Products And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Specifications that limit market access by, for example, favouring one manufacturer to the. Desired product (1) the protein which has the expected structure, or. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. Specification, finished. Assay Limits For Medicinal Products.
From www.ejpps.online
Pharmaceutical Cleanroom Classification using ISO 146441 and the EU Assay Limits For Medicinal Products Objective (1.1) this document is intended to provide guidance regarding good manufacturing. Given pharmaceutical substance or fpp. Desired product (1) the protein which has the expected structure, or. Specifications that limit market access by, for example, favouring one manufacturer to the. (2) the protein which is. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation').. Assay Limits For Medicinal Products.
From www.britannica.com
Enzymelinked immunosorbent assay (ELISA) Definition, Uses, & Method Assay Limits For Medicinal Products And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. (2) the protein which is. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Specifications that limit market access by, for example, favouring. Assay Limits For Medicinal Products.
From www.semanticscholar.org
Table 1 from Inprocess and finished products quality control tests for Assay Limits For Medicinal Products Desired product (1) the protein which has the expected structure, or. (2) the protein which is. Specifications that limit market access by, for example, favouring one manufacturer to the. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Given pharmaceutical substance or fpp. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. This guideline may also. Assay Limits For Medicinal Products.
From www.slideserve.com
PPT Finished Pharmaceutical Product Specifications PowerPoint Assay Limits For Medicinal Products Specification, finished product, chemical, control, test, acceptance criteria, limit, release. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Given pharmaceutical substance or fpp. (2) the protein which is. Desired product (1) the protein which has the expected structure, or. Specifications that limit market access by, for. Assay Limits For Medicinal Products.
From www.pharmapproach.com
Quality Control Tests for Tablets Assay Limits For Medicinal Products (2) the protein which is. Specifications that limit market access by, for example, favouring one manufacturer to the. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. This guideline may also be applicable to other types of products, 43. And/or office of communication, outreach and development center. Assay Limits For Medicinal Products.
From www.americanpharmaceuticalreview.com
Calculating Endotoxin Limits for Drug Products American Assay Limits For Medicinal Products Specifications that limit market access by, for example, favouring one manufacturer to the. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Given pharmaceutical substance or fpp. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. Specification, finished product, chemical, control, test, acceptance criteria, limit, release. And/or office of communication, outreach and. Assay Limits For Medicinal Products.
From www.semanticscholar.org
[PDF] The challenges of potency assay development for cellbased Assay Limits For Medicinal Products Given pharmaceutical substance or fpp. Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). This guideline may also be applicable to other types of products, 43. (2) the protein which is. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Objective (1.1). Assay Limits For Medicinal Products.
From bdn.go.th
10.2 MICROBIAL LIMIT TESTS ตำรายาของประเทศไทย กรมวิทยาศาสตร์การแพทย์ Assay Limits For Medicinal Products Specification, finished product, chemical, control, test, acceptance criteria, limit, release. This guideline may also be applicable to other types of products, 43. (2) the protein which is. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. Specifications that limit market access by, for example, favouring one manufacturer to the. Test methods and validation data, where required (consult. Assay Limits For Medicinal Products.
From pacificbiolabs.com
Compendial Microbiology Pacific BioLabs Assay Limits For Medicinal Products Specifications that limit market access by, for example, favouring one manufacturer to the. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. Given pharmaceutical substance or fpp. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Desired product (1) the protein which has the. Assay Limits For Medicinal Products.
From www.youtube.com
limit Test For Arsenic (As) Pharmaceutical Chemistry YouTube Assay Limits For Medicinal Products Test methods and validation data, where required (consult 'section 7.9 analytical procedures and validation'). Given pharmaceutical substance or fpp. Desired product (1) the protein which has the expected structure, or. Objective (1.1) this document is intended to provide guidance regarding good manufacturing. And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903. Assay Limits For Medicinal Products.
From pharmasciences.in
Uniformity of Dosage Units (UOD) PharmaSciences Assay Limits For Medicinal Products And/or office of communication, outreach and development center for biologics evaluation and research food and drug administration 10903 new hampshire ave., bldg. Specifications that limit market access by, for example, favouring one manufacturer to the. Desired product (1) the protein which has the expected structure, or. This guideline may also be applicable to other types of products, 43. (2) the. Assay Limits For Medicinal Products.
From stabilitystudies.blogspot.com
Pharmaceutical Stability Studies & Method Validations API Assay Limits For Medicinal Products Objective (1.1) this document is intended to provide guidance regarding good manufacturing. This guideline may also be applicable to other types of products, 43. Specifications that limit market access by, for example, favouring one manufacturer to the. (2) the protein which is. Given pharmaceutical substance or fpp. And/or office of communication, outreach and development center for biologics evaluation and research. Assay Limits For Medicinal Products.