Chlorine Or Bromine Better Leaving Group . The equation for a typical nucleophilic substitution reaction. The leaving group is the part of the substrate that is missing at the end of the reaction. The “leaving group” is chlorine, not h+; This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine. Iodine, bromine and chlorine anions are at the top of that list too. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. In order for a leaving group to leave, it must be able to accept electrons. The “original” reaction with a fluoride leaving group, and multiple nitro. Therefore, the leaving group must be a weak base. Then it has a table showing ability to function as leaving group. In addition, iodine is larger and it forms weaker. The computational evidence had suggested it for a while, and experimental research recently caught up. Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. A strong bases wants to donate electrons;
from www.masterorganicchemistry.com
A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Iodine, bromine and chlorine anions are at the top of that list too. A strong bases wants to donate electrons; The computational evidence had suggested it for a while, and experimental research recently caught up. The leaving group is the part of the substrate that is missing at the end of the reaction. Then it has a table showing ability to function as leaving group. The equation for a typical nucleophilic substitution reaction. Therefore, the leaving group must be a weak base. The “original” reaction with a fluoride leaving group, and multiple nitro. This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine.
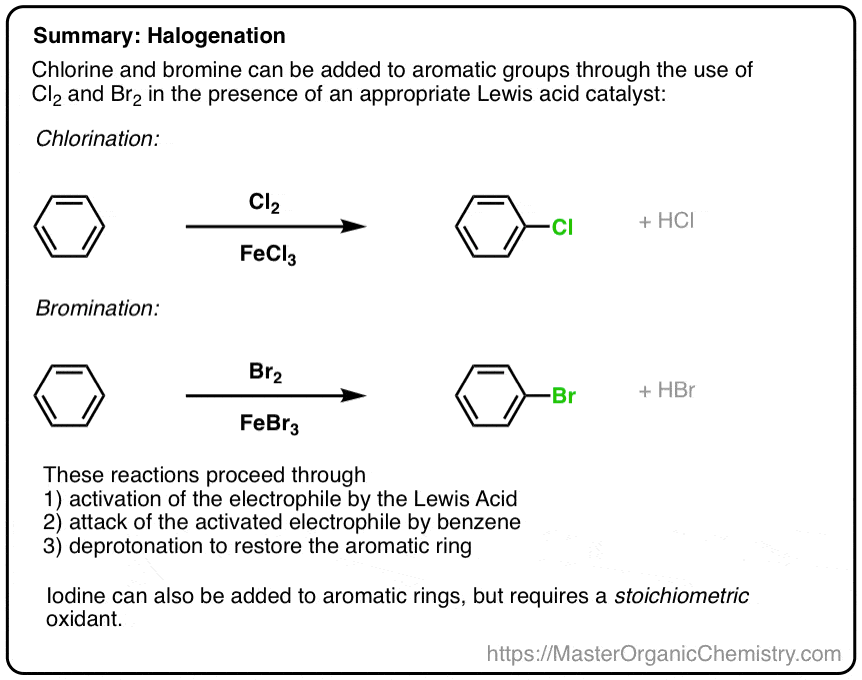
Electrophilic Aromatic Substitutions Chlorination and Bromination
Chlorine Or Bromine Better Leaving Group The equation for a typical nucleophilic substitution reaction. The “leaving group” is chlorine, not h+; In order for a leaving group to leave, it must be able to accept electrons. The computational evidence had suggested it for a while, and experimental research recently caught up. In addition, iodine is larger and it forms weaker. The equation for a typical nucleophilic substitution reaction. Then it has a table showing ability to function as leaving group. The “original” reaction with a fluoride leaving group, and multiple nitro. Therefore, the leaving group must be a weak base. A strong bases wants to donate electrons; A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Iodine, bromine and chlorine anions are at the top of that list too. Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. The leaving group is the part of the substrate that is missing at the end of the reaction. This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine.
From www.masterorganicchemistry.com
Introduction to Free Radical Substitution Reactions Master Organic Chlorine Or Bromine Better Leaving Group Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. A strong bases wants to donate electrons; The “original” reaction with a fluoride leaving group, and multiple nitro. Then it has a table showing ability to function as leaving group. The leaving group is the part of. Chlorine Or Bromine Better Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Chlorine Or Bromine Better Leaving Group The “leaving group” is chlorine, not h+; Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine. Therefore, the leaving group must be a weak base.. Chlorine Or Bromine Better Leaving Group.
From www.youtube.com
Should I Use CHLORINE or BROMINE In My Pool? YouTube Chlorine Or Bromine Better Leaving Group The “original” reaction with a fluoride leaving group, and multiple nitro. In addition, iodine is larger and it forms weaker. A strong bases wants to donate electrons; Iodine, bromine and chlorine anions are at the top of that list too. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Therefore, the. Chlorine Or Bromine Better Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? — Master Organic Chemistry Chlorine Or Bromine Better Leaving Group The computational evidence had suggested it for a while, and experimental research recently caught up. The “leaving group” is chlorine, not h+; A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Then it has a table showing ability to function as leaving group. The “original” reaction with a fluoride leaving group,. Chlorine Or Bromine Better Leaving Group.
From brokeasshome.com
Periodic Table Of Elements Group 17 Chlorine Or Bromine Better Leaving Group The computational evidence had suggested it for a while, and experimental research recently caught up. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. The equation for a typical nucleophilic substitution reaction. In order for a leaving group to leave, it must be able to accept electrons. The leaving group is. Chlorine Or Bromine Better Leaving Group.
From chem.libretexts.org
Hydroxyl Group Substitution Chemistry LibreTexts Chlorine Or Bromine Better Leaving Group This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine. The “original” reaction with a fluoride leaving group, and multiple nitro. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Iodide, which is the least basic of the four common. Chlorine Or Bromine Better Leaving Group.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Chlorine Or Bromine Better Leaving Group Therefore, the leaving group must be a weak base. In addition, iodine is larger and it forms weaker. This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine. Then it has a table showing ability to function as leaving group. The computational evidence had suggested it for a while,. Chlorine Or Bromine Better Leaving Group.
From blog.intheswim.com
Chlorine vs. Bromine What's the Difference? InTheSwim Pool Blog Chlorine Or Bromine Better Leaving Group Iodine, bromine and chlorine anions are at the top of that list too. In addition, iodine is larger and it forms weaker. Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. In order for a leaving group to leave, it must be able to accept electrons.. Chlorine Or Bromine Better Leaving Group.
From www.organicchemistrytutor.com
Substitution and Elimination Reactions — Organic Chemistry Tutor Chlorine Or Bromine Better Leaving Group Iodine, bromine and chlorine anions are at the top of that list too. The leaving group is the part of the substrate that is missing at the end of the reaction. Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. The “leaving group” is chlorine, not. Chlorine Or Bromine Better Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Chlorine Or Bromine Better Leaving Group In order for a leaving group to leave, it must be able to accept electrons. The computational evidence had suggested it for a while, and experimental research recently caught up. This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine. The “original” reaction with a fluoride leaving group, and. Chlorine Or Bromine Better Leaving Group.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Chlorine Or Bromine Better Leaving Group A strong bases wants to donate electrons; A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. The leaving group is the part of the substrate that is missing at the end of the reaction. The “leaving group” is chlorine, not h+; The equation for a typical nucleophilic substitution reaction. Iodide, which. Chlorine Or Bromine Better Leaving Group.
From chem.libretexts.org
Hydroxyl Group Substitution Chemistry LibreTexts Chlorine Or Bromine Better Leaving Group Then it has a table showing ability to function as leaving group. The “original” reaction with a fluoride leaving group, and multiple nitro. A strong bases wants to donate electrons; In addition, iodine is larger and it forms weaker. Iodine, bromine and chlorine anions are at the top of that list too. In order for a leaving group to leave,. Chlorine Or Bromine Better Leaving Group.
From askanydifference.com
Bromin vs Klorin Perbedaan dan Perbandingan Chlorine Or Bromine Better Leaving Group The equation for a typical nucleophilic substitution reaction. Therefore, the leaving group must be a weak base. The leaving group is the part of the substrate that is missing at the end of the reaction. The computational evidence had suggested it for a while, and experimental research recently caught up. The “original” reaction with a fluoride leaving group, and multiple. Chlorine Or Bromine Better Leaving Group.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Chlorine Or Bromine Better Leaving Group The equation for a typical nucleophilic substitution reaction. Then it has a table showing ability to function as leaving group. In addition, iodine is larger and it forms weaker. Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. The “original” reaction with a fluoride leaving group,. Chlorine Or Bromine Better Leaving Group.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes Master Organic Chemistry Chlorine Or Bromine Better Leaving Group Then it has a table showing ability to function as leaving group. In addition, iodine is larger and it forms weaker. A strong bases wants to donate electrons; In order for a leaving group to leave, it must be able to accept electrons. The computational evidence had suggested it for a while, and experimental research recently caught up. A better. Chlorine Or Bromine Better Leaving Group.
From www.doheny.com
Bromine vs. Chlorine Everything You Need to Know Chlorine Or Bromine Better Leaving Group The equation for a typical nucleophilic substitution reaction. This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine. Iodine, bromine and chlorine anions are at the top of that list too. In order for a leaving group to leave, it must be able to accept electrons. The “leaving group”. Chlorine Or Bromine Better Leaving Group.
From easypoolcleaning.com
Bromine Vs Chlorine Hot Tub Sanitizer Which To Choose? Chlorine Or Bromine Better Leaving Group The leaving group is the part of the substrate that is missing at the end of the reaction. Therefore, the leaving group must be a weak base. This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine. The computational evidence had suggested it for a while, and experimental research. Chlorine Or Bromine Better Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Chlorine Or Bromine Better Leaving Group The “original” reaction with a fluoride leaving group, and multiple nitro. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. In addition, iodine is larger and it forms weaker. Therefore, the leaving group must be a weak base. The equation for a typical nucleophilic substitution reaction. A strong bases wants to. Chlorine Or Bromine Better Leaving Group.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference Chlorine Or Bromine Better Leaving Group The equation for a typical nucleophilic substitution reaction. The leaving group is the part of the substrate that is missing at the end of the reaction. Therefore, the leaving group must be a weak base. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. This is one reason why iodide is. Chlorine Or Bromine Better Leaving Group.
From hottubownerhq.com
What's the Difference Between Bromine and Chlorine? Hot Tub Owner HQ Chlorine Or Bromine Better Leaving Group Then it has a table showing ability to function as leaving group. The computational evidence had suggested it for a while, and experimental research recently caught up. The “leaving group” is chlorine, not h+; In order for a leaving group to leave, it must be able to accept electrons. The equation for a typical nucleophilic substitution reaction. A better leaving. Chlorine Or Bromine Better Leaving Group.
From pediaa.com
Difference Between Bromine and Chlorine Chlorine Or Bromine Better Leaving Group In addition, iodine is larger and it forms weaker. A strong bases wants to donate electrons; Then it has a table showing ability to function as leaving group. The leaving group is the part of the substrate that is missing at the end of the reaction. Therefore, the leaving group must be a weak base. Iodide, which is the least. Chlorine Or Bromine Better Leaving Group.
From byjus.com
From each of the following pairs select the compound that will react Chlorine Or Bromine Better Leaving Group Therefore, the leaving group must be a weak base. The “leaving group” is chlorine, not h+; In order for a leaving group to leave, it must be able to accept electrons. The equation for a typical nucleophilic substitution reaction. In addition, iodine is larger and it forms weaker. This is one reason why iodide is a better leaving group than. Chlorine Or Bromine Better Leaving Group.
From quizzlistfestooned.z21.web.core.windows.net
Identify Examples Of Functional Groups Chlorine Or Bromine Better Leaving Group The leaving group is the part of the substrate that is missing at the end of the reaction. Iodine, bromine and chlorine anions are at the top of that list too. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. A strong bases wants to donate electrons; Iodide, which is the. Chlorine Or Bromine Better Leaving Group.
From www.masterorganicchemistry.com
Leaving Group Ability Is Increased By Acid Master Organic Chemistry Chlorine Or Bromine Better Leaving Group Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. In addition, iodine is larger and it forms weaker. In order for a leaving group to leave, it must. Chlorine Or Bromine Better Leaving Group.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Chlorine Or Bromine Better Leaving Group Therefore, the leaving group must be a weak base. In order for a leaving group to leave, it must be able to accept electrons. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. The leaving group is the part of the substrate that is missing at the end of the reaction.. Chlorine Or Bromine Better Leaving Group.
From spasearch.org
Chlorine vs. Bromine Which is Best for Your Home Spa? Chlorine Or Bromine Better Leaving Group The leaving group is the part of the substrate that is missing at the end of the reaction. In order for a leaving group to leave, it must be able to accept electrons. The “original” reaction with a fluoride leaving group, and multiple nitro. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a. Chlorine Or Bromine Better Leaving Group.
From kpu.pressbooks.pub
7.2 SN2 Reaction Mechanisms, Energy Diagram and Stereochemistry Chlorine Or Bromine Better Leaving Group A strong bases wants to donate electrons; The leaving group is the part of the substrate that is missing at the end of the reaction. Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. A better leaving group like bromine, and a weaker electron withdrawing group,. Chlorine Or Bromine Better Leaving Group.
From www.spaworld.com.au
What's better for a spa Chlorine or Bromine? Chlorine Or Bromine Better Leaving Group Therefore, the leaving group must be a weak base. The “leaving group” is chlorine, not h+; In addition, iodine is larger and it forms weaker. The computational evidence had suggested it for a while, and experimental research recently caught up. The equation for a typical nucleophilic substitution reaction. This is one reason why iodide is a better leaving group than. Chlorine Or Bromine Better Leaving Group.
From capoolassociation.com
Which Is Better in a Pool or Spa Chlorine or Bromine? CPA Chlorine Or Bromine Better Leaving Group The “leaving group” is chlorine, not h+; Iodine, bromine and chlorine anions are at the top of that list too. A strong bases wants to donate electrons; Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. This is one reason why iodide is a better leaving. Chlorine Or Bromine Better Leaving Group.
From epichottubs.com
Bromine or Chlorine Which One is Best for Your Hot Tub? Chlorine Or Bromine Better Leaving Group The “leaving group” is chlorine, not h+; The leaving group is the part of the substrate that is missing at the end of the reaction. Iodine, bromine and chlorine anions are at the top of that list too. Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among. Chlorine Or Bromine Better Leaving Group.
From www.chegg.com
Solved 2. Chlorine or bromine related reaction Chlorine Or Bromine Better Leaving Group The “original” reaction with a fluoride leaving group, and multiple nitro. The “leaving group” is chlorine, not h+; A strong bases wants to donate electrons; Then it has a table showing ability to function as leaving group. This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine. In order. Chlorine Or Bromine Better Leaving Group.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Chlorine Or Bromine Better Leaving Group The leaving group is the part of the substrate that is missing at the end of the reaction. Therefore, the leaving group must be a weak base. The “original” reaction with a fluoride leaving group, and multiple nitro. This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine. Iodide,. Chlorine Or Bromine Better Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Chlorine Or Bromine Better Leaving Group Iodide, which is the least basic of the four common halides (f, cl, br, and i), is the best leaving group among them. Iodine, bromine and chlorine anions are at the top of that list too. Therefore, the leaving group must be a weak base. The “leaving group” is chlorine, not h+; In order for a leaving group to leave,. Chlorine Or Bromine Better Leaving Group.
From www.chegg.com
Solved I know Bromine is a better leaving group than Chlorine Or Bromine Better Leaving Group Therefore, the leaving group must be a weak base. In order for a leaving group to leave, it must be able to accept electrons. This is one reason why iodide is a better leaving group than bromide, even though bromine is more electronegative than iodine. A strong bases wants to donate electrons; Iodine, bromine and chlorine anions are at the. Chlorine Or Bromine Better Leaving Group.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Chlorine Or Bromine Better Leaving Group Iodine, bromine and chlorine anions are at the top of that list too. The “leaving group” is chlorine, not h+; The leaving group is the part of the substrate that is missing at the end of the reaction. The equation for a typical nucleophilic substitution reaction. A better leaving group like bromine, and a weaker electron withdrawing group, tends to. Chlorine Or Bromine Better Leaving Group.