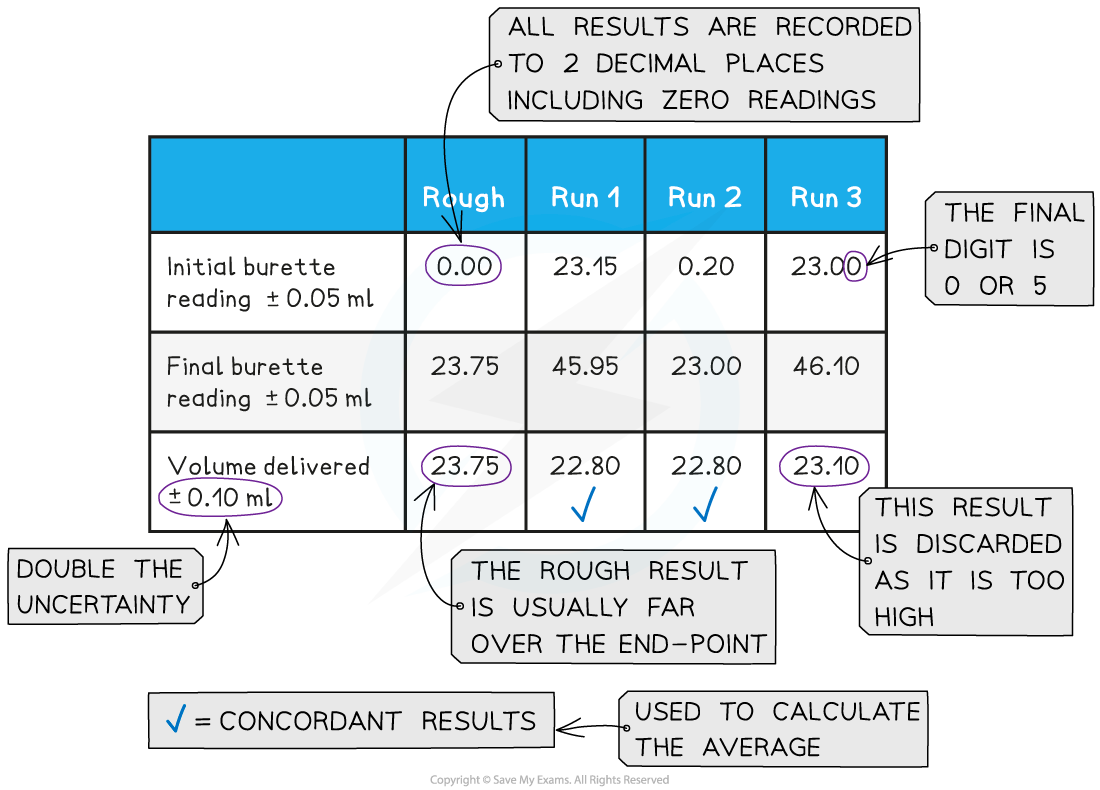
End Point Of Titration Mean . A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. For instance, you might add a standard base solution to an mystery acid solution. The end point is where the titration ends in practice. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration measures the concentration of an unknown solution that reacts with a solution of known. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. As the addition takes place, the two reagents in. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Using a ph probe and using a colour indicator. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titrations with a ph probe. To successfully monitor the titration’s end point using an indicator or a ph probe, the titrand needs an initial volume of approximately 25 ml. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: If we assume the analyte’s formula.
from www.hanlin.com
Titration measures the concentration of an unknown solution that reacts with a solution of known. Using a ph probe and using a colour indicator. As the addition takes place, the two reagents in. For instance, you might add a standard base solution to an mystery acid solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titrations with a ph probe. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. For a ph titration (between an acid and a base), there are two common ways to find the endpoint:
Edexcel A Level Chemistry复习笔记1.7.2 Titrations翰林国际教育
End Point Of Titration Mean As the addition takes place, the two reagents in. The end point is where the titration ends in practice. Using a ph probe and using a colour indicator. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. To successfully monitor the titration’s end point using an indicator or a ph probe, the titrand needs an initial volume of approximately 25 ml. For instance, you might add a standard base solution to an mystery acid solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. Titrations with a ph probe. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. If we assume the analyte’s formula. As the addition takes place, the two reagents in. Titration measures the concentration of an unknown solution that reacts with a solution of known.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 End Point Of Titration Mean If we assume the analyte’s formula. The end point is where the titration ends in practice. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The closer the end point to the equivalence point the better, but it is often not easy to find a good. End Point Of Titration Mean.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps End Point Of Titration Mean The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown. End Point Of Titration Mean.
From www.hotzxgirl.com
Titration Curve Labeled Hot Sex Picture End Point Of Titration Mean If we assume the analyte’s formula. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. Titration measures the concentration of an unknown solution that reacts with a solution of known. Using a ph probe and using a colour indicator. Titration involves the gradual addition of a. End Point Of Titration Mean.
From www.youtube.com
Thermometric Titration YouTube End Point Of Titration Mean Using a ph probe and using a colour indicator. Titrations with a ph probe. To successfully monitor the titration’s end point using an indicator or a ph probe, the titrand needs an initial volume of approximately 25 ml. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process. End Point Of Titration Mean.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic End Point Of Titration Mean If we assume the analyte’s formula. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. Using a ph probe and using a colour indicator. To successfully monitor the titration’s end point using an indicator or a ph probe, the titrand needs an initial volume of approximately. End Point Of Titration Mean.
From gioiuscok.blob.core.windows.net
Titration Management Meaning at Joanne Bowen blog End Point Of Titration Mean A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. As the addition takes place, the two reagents in. Titration involves the gradual addition of a reagent of. End Point Of Titration Mean.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记1.7.2 Titrations翰林国际教育 End Point Of Titration Mean The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. For instance, you might add a standard base solution to an mystery acid solution. Titrations with a ph probe. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. End Point Of Titration Mean.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download End Point Of Titration Mean Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. As the addition takes place, the two reagents in. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. To. End Point Of Titration Mean.
From exovodadv.blob.core.windows.net
Titration End Point Meaning at Jenny Ingram blog End Point Of Titration Mean A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titrations with a ph probe. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The. End Point Of Titration Mean.
From studydystrophic.z4.web.core.windows.net
How To Find Equivalence Point On Excel Graph End Point Of Titration Mean For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. As the addition takes place, the two reagents in. The end point is where the titration ends in. End Point Of Titration Mean.
From theedge.com.hk
Chemistry How To Titration The Edge End Point Of Titration Mean Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: As the addition takes place, the two reagents in. If we assume the analyte’s formula. The closer the end point to the equivalence point the better, but it is often not easy. End Point Of Titration Mean.
From gioazgcfn.blob.core.windows.net
Titration Meaning In Medical Term at Mary Graham blog End Point Of Titration Mean The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. For instance, you might add a standard base solution to an mystery acid solution. Titration measures the concentration of an unknown solution that reacts with a solution of known. A point of equivalence in a titration refers. End Point Of Titration Mean.
From exovodadv.blob.core.windows.net
Titration End Point Meaning at Jenny Ingram blog End Point Of Titration Mean The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. As the addition takes place, the two reagents in. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. End Point Of Titration Mean.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts End Point Of Titration Mean For a ph titration (between an acid and a base), there are two common ways to find the endpoint: As the addition takes place, the two reagents in. For instance, you might add a standard base solution to an mystery acid solution. The closer the end point to the equivalence point the better, but it is often not easy to. End Point Of Titration Mean.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog End Point Of Titration Mean For instance, you might add a standard base solution to an mystery acid solution. Using a ph probe and using a colour indicator. To successfully monitor the titration’s end point using an indicator or a ph probe, the titrand needs an initial volume of approximately 25 ml. Titration measures the concentration of an unknown solution that reacts with a solution. End Point Of Titration Mean.
From www.numerade.com
SOLVED RGiSTLABORATORY QUESTIONS AcidBase Titration of Polyprotic End Point Of Titration Mean As the addition takes place, the two reagents in. For instance, you might add a standard base solution to an mystery acid solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The closer the end point to. End Point Of Titration Mean.
From hxeptiewa.blob.core.windows.net
Find Equivalence Point From Titration at Roger Cornelius blog End Point Of Titration Mean For instance, you might add a standard base solution to an mystery acid solution. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves. End Point Of Titration Mean.
From mungfali.com
Acid Base Titration Method End Point Of Titration Mean Titration measures the concentration of an unknown solution that reacts with a solution of known. To successfully monitor the titration’s end point using an indicator or a ph probe, the titrand needs an initial volume of approximately 25 ml. If we assume the analyte’s formula. For a ph titration (between an acid and a base), there are two common ways. End Point Of Titration Mean.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS End Point Of Titration Mean A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. As the addition takes place, the two reagents in. Titrations with a ph probe. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second. End Point Of Titration Mean.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki End Point Of Titration Mean A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. For a ph titration (between an acid and a base), there are two common ways to find the. End Point Of Titration Mean.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts End Point Of Titration Mean For instance, you might add a standard base solution to an mystery acid solution. Titrations with a ph probe. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different. End Point Of Titration Mean.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS End Point Of Titration Mean Titration measures the concentration of an unknown solution that reacts with a solution of known. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. To successfully monitor the titration’s end point using an indicator or a ph probe, the titrand needs an initial volume of approximately. End Point Of Titration Mean.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co End Point Of Titration Mean A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The end point is where the titration ends in practice. If we assume the analyte’s formula. Titration measures the concentration of an unknown solution that reacts with a solution of known. The closer the end point to. End Point Of Titration Mean.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts End Point Of Titration Mean For instance, you might add a standard base solution to an mystery acid solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titrations with a ph. End Point Of Titration Mean.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare End Point Of Titration Mean The end point is where the titration ends in practice. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. If we assume the analyte’s formula. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. End Point Of Titration Mean.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre End Point Of Titration Mean The end point is where the titration ends in practice. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Using a ph probe and using a colour. End Point Of Titration Mean.
From mmerevise.co.uk
Titrations and Uncertainties MME End Point Of Titration Mean For instance, you might add a standard base solution to an mystery acid solution. Titration measures the concentration of an unknown solution that reacts with a solution of known. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The end point is where the titration ends. End Point Of Titration Mean.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts End Point Of Titration Mean A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a. End Point Of Titration Mean.
From exoxoimsp.blob.core.windows.net
Point Titration Definition at Gerald Lathrop blog End Point Of Titration Mean Titration measures the concentration of an unknown solution that reacts with a solution of known. To successfully monitor the titration’s end point using an indicator or a ph probe, the titrand needs an initial volume of approximately 25 ml. As the addition takes place, the two reagents in. If we assume the analyte’s formula. A titration is a laboratory technique. End Point Of Titration Mean.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple End Point Of Titration Mean For instance, you might add a standard base solution to an mystery acid solution. The end point is where the titration ends in practice. Titration measures the concentration of an unknown solution that reacts with a solution of known. If we assume the analyte’s formula. Using a ph probe and using a colour indicator. To successfully monitor the titration’s end. End Point Of Titration Mean.
From www.youtube.com
What is the difference between the endpoint and equivalence point in a End Point Of Titration Mean Titration measures the concentration of an unknown solution that reacts with a solution of known. To successfully monitor the titration’s end point using an indicator or a ph probe, the titrand needs an initial volume of approximately 25 ml. For instance, you might add a standard base solution to an mystery acid solution. Titrations with a ph probe. The closer. End Point Of Titration Mean.
From www.numerade.com
SOLVED RGiSTLABORATORY QUESTIONS AcidBase Titration of Polyprotic End Point Of Titration Mean To successfully monitor the titration’s end point using an indicator or a ph probe, the titrand needs an initial volume of approximately 25 ml. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the. End Point Of Titration Mean.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple End Point Of Titration Mean For instance, you might add a standard base solution to an mystery acid solution. If we assume the analyte’s formula. As the addition takes place, the two reagents in. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titrations with a ph. End Point Of Titration Mean.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors End Point Of Titration Mean The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. The end point is where the titration ends in practice. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. End Point Of Titration Mean.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube End Point Of Titration Mean For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different. End Point Of Titration Mean.