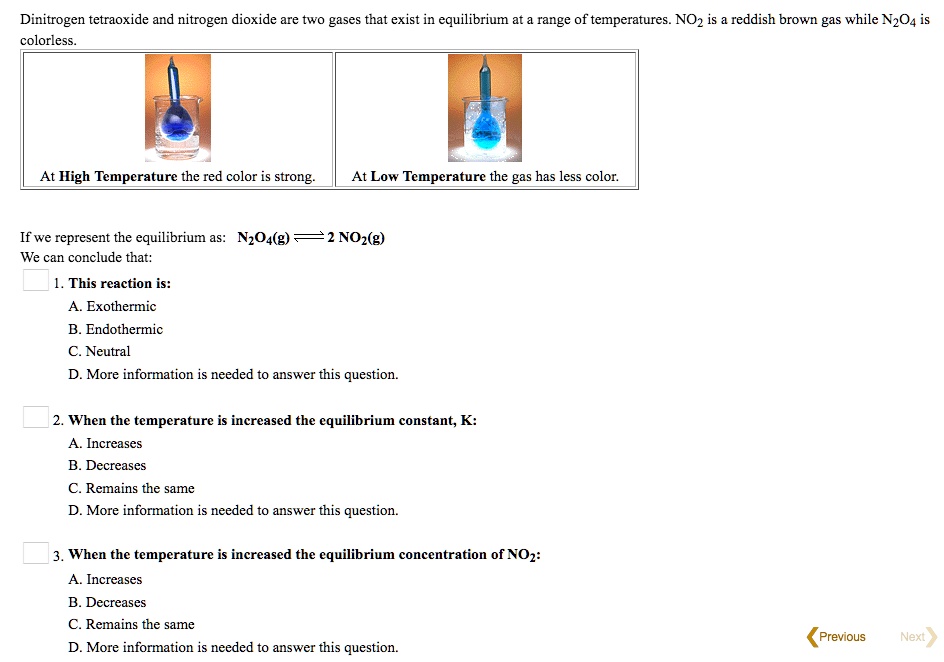
Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium . On its own, this is. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Plot graphs of volume against temperature for both gases on the same axes. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. At equilibrium, both gases are present in. Nitrogen dioxide, no 2, is a dark brown gas. Continue taking readings until the temperature is between 70 to 80 °c. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ).
from www.numerade.com
Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Continue taking readings until the temperature is between 70 to 80 °c. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). Plot graphs of volume against temperature for both gases on the same axes. On its own, this is. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. At equilibrium, both gases are present in. Nitrogen dioxide, no 2, is a dark brown gas.
SOLVED Dinitrogen tetraoxide and nitrogen dioxide are two gases that
Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium On its own, this is. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. At equilibrium, both gases are present in. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. Nitrogen dioxide, no 2, is a dark brown gas. Continue taking readings until the temperature is between 70 to 80 °c. On its own, this is. Plot graphs of volume against temperature for both gases on the same axes. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ).
From www.chegg.com
Solved nitrogen dioxide gas reacts with nitrogen trioxide Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium On its own, this is. At equilibrium, both gases are present in. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). Dinitrogen tetroxide, n 2 o 4, is formed when. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
The effect of pressure and temperature on equilibrium Le Chatelier's Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Nitrogen dioxide, no 2, is a dark brown gas. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). Plot graphs of volume against. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From fphoto.photoshelter.com
science chemical oxidation reaction nitrogen oxides Fundamental Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). At equilibrium, both gases are present in. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. Continue taking readings until the temperature is between 70 to 80 °c. Plot graphs of volume against temperature. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.mdpi.com
Applied Sciences Free FullText Nitrogen Dioxide Gas Levels in TBM Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Nitrogen dioxide, no 2, is a dark brown gas. Plot graphs of volume against temperature for both gases on the same axes. Um demonstrationnitrogen dioxide gas. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.numerade.com
SOLVED The conversion of nitrogen dioxide gas into dinitrogen Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. The equilibrium illustrated in. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.slideserve.com
PPT The NAAQS The Dirty Half Dozen PowerPoint Presentation, free Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. Plot graphs of volume against temperature for both gases on the same axes. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.numerade.com
SOLVEDAt a certain temperature, nitrogen and oxygen gases combine to Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium At equilibrium, both gases are present in. Plot graphs of volume against temperature for both gases on the same axes. Nitrogen dioxide, no 2, is a dark brown gas. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From ar.inspiredpencil.com
Nitrogen Dioxide Gas Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. On its own, this is. Nitrogen dioxide, no 2, is a dark brown gas.. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From 2012books.lardbucket.org
Chemical Equilibrium Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. Continue taking readings until the temperature is between 70 to 80 °c. At equilibrium, both gases are present in. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). Nitrogen dioxide gas is dark brown in color and remains. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.numerade.com
Nitrogen dioxide is a redbrown gas that is responsible for the color Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium On its own, this is. Plot graphs of volume against temperature for both gases on the same axes. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Dinitrogen. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.youtube.com
Nitrogen Dioxide and Dinitrogen Tetroxide Equilibrium // HSC Chemistry Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium At equilibrium, both gases are present in. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. On its own, this is. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. Nitrogen dioxide, no 2, is a dark brown gas. Plot graphs of volume against temperature for both gases on the same axes.. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.numerade.com
SOLVED Dinitrogen tetraoxide and nitrogen dioxide are two gases that Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. Continue taking readings until the temperature is between 70 to 80 °c. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.numerade.com
SOLVED In the reaction of ammonia gas with oxygen, nitrogen dioxide Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium At equilibrium, both gases are present in. On its own, this is. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Nitrogen dioxide gas is dark brown in color and remains in. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.chegg.com
Solved Nitrogen dioxide is one of the many oxides of Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Nitrogen dioxide, no 2, is a dark brown gas. On its own, this is. At equilibrium, both gases are. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.dreamstime.com
Nitrogen Dioxide Cloud Stock Photos Free & RoyaltyFree Stock Photos Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. At equilibrium, both gases are present in. On its own, this is. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.youtube.com
Colorless dinitrogen tetroxide N2O4 in equilibrium with dark brown Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. On its own, this is. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). Continue taking readings until the temperature is between 70 to 80 °c. Um. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.sciencephoto.com
Nitrogen Dioxide Equilibrium Stock Image C002/8226 Science Photo Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Continue taking readings until the temperature is between 70 to 80 °c. On its own, this is. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Plot graphs of volume against temperature for both gases on the same axes. Um demonstrationnitrogen dioxide gas is used to demonstrate. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From commons.wikimedia.org
FileNitrogendioxide3DvdW.png Wikimedia Commons Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Plot graphs of volume against temperature for both gases on the same axes. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. At equilibrium, both gases are present in. Um demonstrationnitrogen dioxide gas is used. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.chegg.com
Solved 8 Gaseous dinitrogen tetroxide, N2O4, and nitrogen Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. On its own, this is. Nitrogen dioxide, no 2, is a dark brown gas. Continue taking readings until the temperature is between 70 to 80 °c.. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From fphoto.photoshelter.com
science chemical oxidation reaction nitrogen oxides Fundamental Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Plot graphs of volume against temperature for both gases on the same axes. On its own, this is. Nitrogen dioxide, no 2, is a dark brown gas. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. At equilibrium, both gases are present in. The equilibrium illustrated in this demonstration is. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From fphoto.photoshelter.com
science chemical oxidation reaction nitrogen oxides Fundamental Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium On its own, this is. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Nitrogen dioxide, no 2, is a dark. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From fphoto.photoshelter.com
science chemistry gases bromine Fundamental Photographs The Art of Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. Continue taking readings until the temperature is between 70 to 80 °c. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Plot graphs of volume against temperature for both gases on the same axes. At equilibrium,. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.chegg.com
Solved Nitrogen dioxide is a redbrown gas that is Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). On its own, this is. Continue taking readings until the temperature is between 70 to 80 °c. Nitrogen dioxide, no 2,. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.chegg.com
Solved Nitrogen dioxide is one of the many oxides of Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. On its own, this is. Plot graphs of volume against temperature for both gases on the same axes. Continue taking readings until the temperature is between 70 to 80 °c. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.nagwa.com
Question Video Identifying the Color Change When Nitrogen Monoxide Gas Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). Plot graphs of volume against temperature for both gases on. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.vecteezy.com
Cylinders with different types of liquid gas. Container balloon gas Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Plot graphs of volume against temperature for both gases on the same axes. Continue taking readings until the temperature is between 70 to 80 °c. On its own, this is. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). Nitrogen dioxide gas is dark brown in color and remains. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.youtube.com
Chemical Equilibrium between N2O4 (colorless gas) and NO2 (brown gas Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Nitrogen dioxide, no 2, is a dark brown gas. Plot graphs of volume against temperature for both gases on the same axes. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. The. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.numerade.com
SOLVED Dinitrogen tetraoxide is colorless gas that dissociates into Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Plot graphs of volume against temperature for both gases on the same axes. Continue taking readings until the temperature is between 70 to 80 °c. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Nitrogen dioxide, no 2, is a dark brown gas. Dinitrogen tetroxide, n 2 o 4, is. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.numerade.com
SOLVED You start with 100mL of 14M HNO3 and 10grams of copper metal Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium At equilibrium, both gases are present in. On its own, this is. Nitrogen dioxide, no 2, is a dark brown gas. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Plot graphs of volume against temperature for both gases on the same axes. Continue taking readings until the temperature is. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.chemistrystudent.com
Alkanes (ALevel) ChemistryStudent Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. At equilibrium, both gases are present in. On its own, this is. Plot graphs of volume against temperature for both gases on the same axes. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. The changing color of $\ce{no_2}$. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From stock.adobe.com
Nitric oxide, nitrogen dioxide is a poisonous gas, reddishbrown in Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Plot graphs of volume against temperature for both gases on the same axes. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). Nitrogen dioxide, no 2, is a dark brown gas. On its own, this is. At equilibrium, both gases are present in. Nitrogen dioxide gas is dark brown. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.alamy.com
Nitrogen dioxide (NO2) is a reddishbrown gas that belongs to the Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Continue taking readings until the temperature is between 70 to 80 °c. Nitrogen dioxide, no 2, is a dark brown gas. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.chegg.com
Solved Dinitrogen tetraoxide is a colorless gas that Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Plot graphs of volume against temperature for both gases on the same axes. Um demonstrationnitrogen dioxide gas is used to demonstrate le chatelier’s principl. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From fphoto.photoshelter.com
equilibrium nitrogen dioxide labglass Fundamental Photographs The Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium On its own, this is. Dinitrogen tetroxide, n 2 o 4, is formed when two no 2 molecules join together. The equilibrium illustrated in this demonstration is between nitrogen dioxide (no 2) and dinitrogen tetroxide (n 2 o 4 ). Nitrogen dioxide, no 2, is a dark brown gas. At equilibrium, both gases are present in. Plot graphs of volume. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.
From www.youtube.com
How to Balance N2 + O2 = NO2 (Nitrogen gas + Oxygen gas) YouTube Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium Continue taking readings until the temperature is between 70 to 80 °c. The changing color of $\ce{no_2}$ is because it exits in equilibrium with $\ce{n_2o_4}$ as shown in the following equation $$\ce{2 no_2 <=>. Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless. Nitrogen dioxide, no 2, is a dark. Nitrogen Dioxide Gas Is Dark Brown In Color And Remains In Equilibrium.