Chlorine Charge Atom . 93 rows this table shows the most common charges for atoms of the chemical elements. Uncombined elements have an oxidation state of 0. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. You can use this table to predict whether an atom can bond with another atom. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. It is defined as being the charge that an atom would have if all bonds were ionic.
from periodictableguide.com
It is defined as being the charge that an atom would have if all bonds were ionic. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. 93 rows this table shows the most common charges for atoms of the chemical elements. Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. You can use this table to predict whether an atom can bond with another atom. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Uncombined elements have an oxidation state of 0. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed.
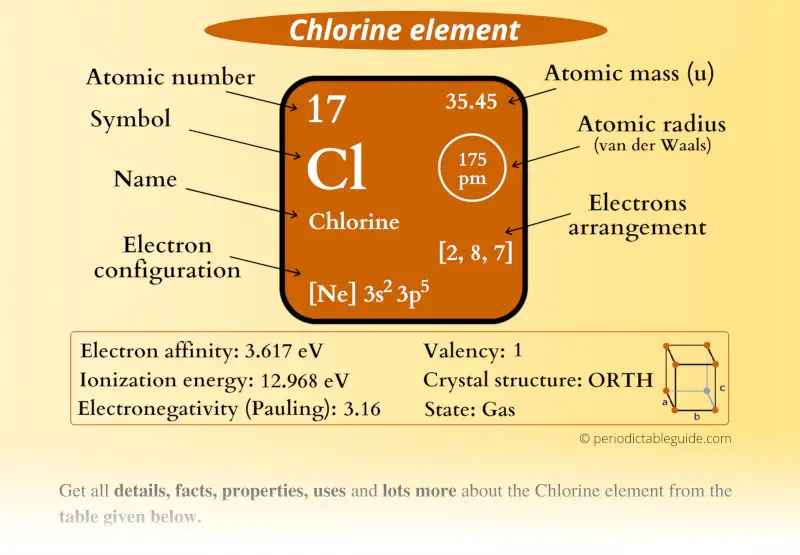
Chlorine (Cl) Periodic Table (Element Information & More)
Chlorine Charge Atom It is defined as being the charge that an atom would have if all bonds were ionic. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. You can use this table to predict whether an atom can bond with another atom. 93 rows this table shows the most common charges for atoms of the chemical elements. Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Charge Atom Uncombined elements have an oxidation state of 0. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. It is defined as being the charge that an atom would have if. Chlorine Charge Atom.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Charge Atom You can use this table to predict whether an atom can bond with another atom. 93 rows this table shows the most common charges for atoms of the chemical elements. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Uncombined elements have an oxidation state of 0. 93 rows when atoms. Chlorine Charge Atom.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Charge Atom Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows this. Chlorine Charge Atom.
From slideplayer.com
What are ionic compounds and how do they form? ppt download Chlorine Charge Atom Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. 93 rows this table shows the most common charges for atoms of the chemical elements. It is defined as being the charge that an atom would have if all bonds were ionic. You can use this table to predict. Chlorine Charge Atom.
From www.vrogue.co
Orbital Diagram For Chlorine vrogue.co Chlorine Charge Atom Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It is defined as being the charge that an atom would have if all bonds were ionic. Chlorine is a chemical. Chlorine Charge Atom.
From favpng.com
Lewis Structure Chlorine Chloride Electron Diagram, PNG, 600x600px Chlorine Charge Atom When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. 93 rows this table shows the most common charges for atoms of the chemical elements. Chlorine reacts with most metals and. Chlorine Charge Atom.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Chlorine Charge Atom Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows this table shows the most common charges for atoms of the chemical elements. When these atoms gain electrons, they acquire a negative charge. Chlorine Charge Atom.
From brokeasshome.com
Periodic Table Chlorine Electrons Chlorine Charge Atom 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given. Chlorine Charge Atom.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C023/2480 Science Photo Chlorine Charge Atom Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. 93 rows this table shows the most common charges for atoms of the chemical elements. Chlorine reacts with most metals and. Chlorine Charge Atom.
From periodictableguide.com
Chlorine (Cl) Periodic Table (Element Information & More) Chlorine Charge Atom Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. You can use this table to predict whether an atom can bond with another atom. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. 93 rows this table shows the. Chlorine Charge Atom.
From sciencenotes.org
Chlorine Facts Chlorine Charge Atom Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.. Chlorine Charge Atom.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Charge Atom Uncombined elements have an oxidation state of 0. Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. You can use this table to predict whether an atom can bond with another atom. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the. Chlorine Charge Atom.
From www.vectorstock.com
Atom chlorine Royalty Free Vector Image VectorStock Chlorine Charge Atom Uncombined elements have an oxidation state of 0. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. 93 rows this table shows the most common charges for atoms of the chemical elements. Total number of protons in the nucleus is called the atomic number of. Chlorine Charge Atom.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Chlorine Charge Atom When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were ionic. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its. Chlorine Charge Atom.
From www.shutterstock.com
3.722 Atom structure of chlorine Görseli, Stok Fotoğraflar ve Vektörler Chlorine Charge Atom Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. You can use this table to. Chlorine Charge Atom.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Charge Atom Uncombined elements have an oxidation state of 0. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows this table shows the most common charges for atoms of the chemical. Chlorine Charge Atom.
From circuitdataboattrains.z14.web.core.windows.net
Chlorine Atom Diagram Chlorine Charge Atom 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. 93 rows this table shows the most common charges for atoms of the chemical elements.. Chlorine Charge Atom.
From www.chegg.com
Solved What is the formal charge of the chlorine atom in Chlorine Charge Atom Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. 93 rows this. Chlorine Charge Atom.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Charge Atom Uncombined elements have an oxidation state of 0. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the. Chlorine Charge Atom.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Charge Atom Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. It is defined as being the charge that an atom would have if all bonds were ionic. Chlorine is a chemical. Chlorine Charge Atom.
From gardenandplate.com
Molecules Chlorine Charge Atom 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. You can use this table to predict whether an atom can bond with another atom. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. When these atoms. Chlorine Charge Atom.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Charge Atom 93 rows this table shows the most common charges for atoms of the chemical elements. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z.. Chlorine Charge Atom.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Charge Atom When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. It is defined as being the charge that an atom would have if all bonds were ionic. Chlorine is a chemical element with atomic. Chlorine Charge Atom.
From delorescgough.blob.core.windows.net
Chlorine Electron Charge at delorescgough blog Chlorine Charge Atom You can use this table to predict whether an atom can bond with another atom. Uncombined elements have an oxidation state of 0. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Total number of protons in the nucleus is called the atomic number of the atom and is given the. Chlorine Charge Atom.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Charge Atom Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. 93 rows when atoms gain electron/s,. Chlorine Charge Atom.
From www.dreamstime.com
An Atom of Chlorine Diagram Stock Vector Illustration of structure Chlorine Charge Atom 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water.. Chlorine Charge Atom.
From slideplayer.com
Illustrating the bonding of Sodium and Chlorine ppt download Chlorine Charge Atom Uncombined elements have an oxidation state of 0. You can use this table to predict whether an atom can bond with another atom. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. When these atoms gain electrons, they acquire a negative charge because they now. Chlorine Charge Atom.
From www.dreamstime.com
Chlorine Atom, with Mass and Energy Levels. Stock Vector Illustration Chlorine Charge Atom When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. 93 rows this table shows the most common charges for atoms of the chemical elements. Chlorine is a chemical element with. Chlorine Charge Atom.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Charge Atom You can use this table to predict whether an atom can bond with another atom. Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. 93 rows this table shows the most common charges for atoms of the chemical elements. It is defined as being the charge that an atom would have. Chlorine Charge Atom.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Charge Atom Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It is defined as being the charge that an atom would have if all bonds were ionic. Uncombined elements have an. Chlorine Charge Atom.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Charge Atom It is defined as being the charge that an atom would have if all bonds were ionic. Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. You can use this table to predict. Chlorine Charge Atom.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Charge Atom Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom. Chlorine Charge Atom.
From brainly.com
The diagram shows the electron configuration around the chlorine Chlorine Charge Atom You can use this table to predict whether an atom can bond with another atom. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. When these atoms. Chlorine Charge Atom.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Charge Atom Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows when atoms gain electron/s,. Chlorine Charge Atom.
From mavink.com
Bohr Model Of Chlorine Chlorine Charge Atom When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged. Chlorine Charge Atom.