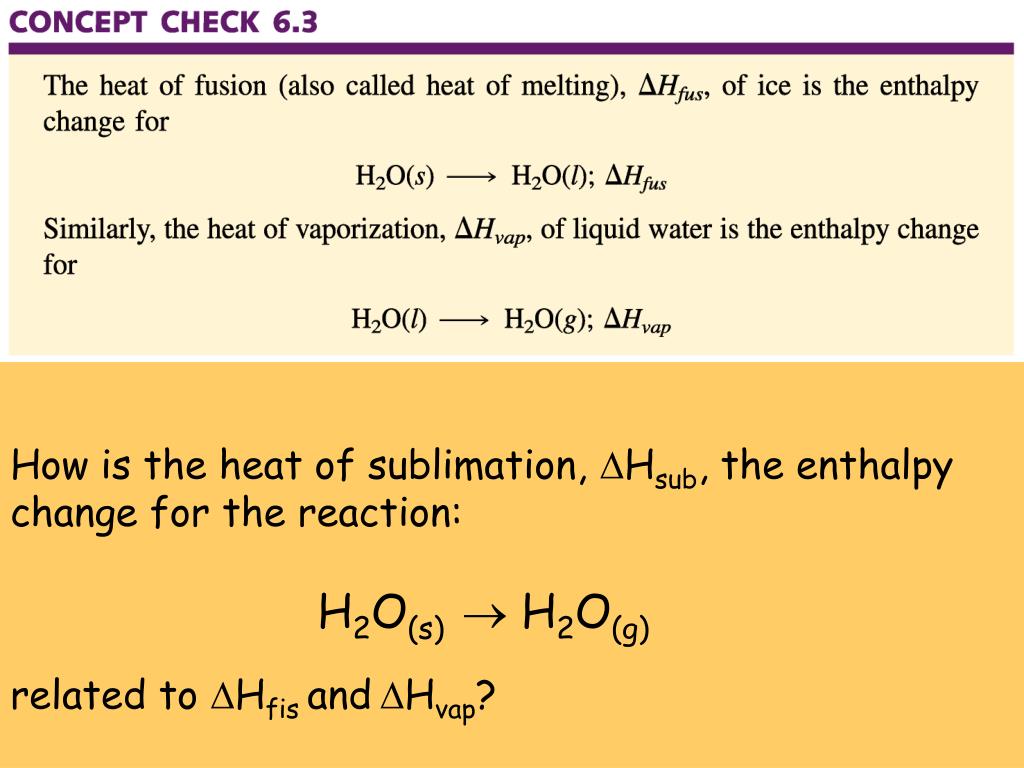
Standard Enthalpy Change Of Formation Zero . The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. Elements in their standard state are not formed, they just are. The standard enthalpy of formation of any element in its standard state is zero by definition. Why is that a convention? The standard enthalpy of formation for an element in its standard state is zero!!!! By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Oxygen (the element) at standard state is o 2. \[\delta h_{reaction}^o = \sum {\delta. To find the δh reaction o, use the formula for the standard enthalpy change of formation:
from www.slideserve.com
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Elements in their standard state are not formed, they just are. The standard enthalpy of formation for an element in its standard state is zero!!!! The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Why is that a convention? To find the δh reaction o, use the formula for the standard enthalpy change of formation: The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. The standard enthalpy of formation of any element in its standard state is zero by definition.
PPT Standard Enthalpies of Formation PowerPoint Presentation, free
Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation for an element in its standard state is zero!!!! Elements in their standard state are not formed, they just are. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. \[\delta h_{reaction}^o = \sum {\delta. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Why is that a convention? To find the δh reaction o, use the formula for the standard enthalpy change of formation: The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Oxygen (the element) at standard state is o 2.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation for elements. Standard Enthalpy Change Of Formation Zero.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation of any element in its standard state is zero by definition. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. \[\delta h_{reaction}^o = \sum {\delta. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero. Standard Enthalpy Change Of Formation Zero.
From rmcstudy.ie
rmcstudy Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation of any element in its standard state is zero by definition. Oxygen (the element) at standard state is o 2. \[\delta h_{reaction}^o = \sum {\delta. The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of formation for elements in their most stable form is defined as zero,. Standard Enthalpy Change Of Formation Zero.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Change Of Formation Zero \[\delta h_{reaction}^o = \sum {\delta. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving. Standard Enthalpy Change Of Formation Zero.
From slideplayer.com
Standard Enthalpies of Formation ppt download Standard Enthalpy Change Of Formation Zero By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Elements in their standard state are not formed, they just are. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation $\delta. Standard Enthalpy Change Of Formation Zero.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Change Of Formation Zero The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation of any element in its standard state. Standard Enthalpy Change Of Formation Zero.
From www.msjchem.com
Topic 5 Energetics MSJChem Tutorial videos for IB Chemistry Standard Enthalpy Change Of Formation Zero The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. Why is that a convention? By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. To find the δh reaction o, use. Standard Enthalpy Change Of Formation Zero.
From zeviernswenson.blogspot.com
Standard Enthalpy of Formation ZeviernSwenson Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation for an element in its standard state is zero!!!! Oxygen (the element) at standard state is o 2.. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation for an element in its standard state is zero!!!! 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Why is that a convention? The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as. Standard Enthalpy Change Of Formation Zero.
From scienceinfo.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance Standard Enthalpy Change Of Formation Zero For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Why is that a convention? 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation of any element. Standard Enthalpy Change Of Formation Zero.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Change Of Formation Zero \[\delta h_{reaction}^o = \sum {\delta. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT Energetics PowerPoint Presentation ID1204656 Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation for an element in its standard state is zero!!!! Why is that a convention? The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The same is true. Standard Enthalpy Change Of Formation Zero.
From www.chegg.com
Solved Calculate the Standard Enthalpy of Formation of NOCl Standard Enthalpy Change Of Formation Zero By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving. Standard Enthalpy Change Of Formation Zero.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation for an element in its standard state is zero!!!! \[\delta h_{reaction}^o = \sum {\delta. Why is that a convention? Oxygen (the element) at standard state is. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Change Of Formation Zero The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation for an element in its standard state is zero!!!! For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. \[\delta h_{reaction}^o = \sum {\delta. Why is that a convention? The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. To find the δh reaction o, use. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Change Of Formation Zero The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. Why is that a convention? To find the δh reaction o, use the formula for the standard enthalpy change of formation: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Enthalpy Change Of Formation Zero.
From www.youtube.com
15.1.1 Define Standard State, Enthalpy change of formation and Standard Enthalpy Change Of Formation Zero Elements in their standard state are not formed, they just are. Why is that a convention? Oxygen (the element) at standard state is o 2. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of formation for. Standard Enthalpy Change Of Formation Zero.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Change Of Formation Zero Why is that a convention? 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. \[\delta h_{reaction}^o = \sum {\delta. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard Enthalpy Change Of Formation Zero.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Change Of Formation Zero Oxygen (the element) at standard state is o 2. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. To find the δh reaction o, use the formula for the standard enthalpy. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Change Of Formation Zero 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its.. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Change Of Formation Zero Why is that a convention? The standard enthalpy of formation of any element in its standard state is zero by definition. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. To find the δh reaction o, use the formula for the standard. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5072129 Standard Enthalpy Change Of Formation Zero The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The standard enthalpy of formation for elements in their. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID3196359 Standard Enthalpy Change Of Formation Zero By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation for an element in its standard state is zero!!!! Oxygen (the element) at standard state is o 2. The standard enthalpy of formation $\delta h_f^°$ of pure elements. Standard Enthalpy Change Of Formation Zero.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Change Of Formation Zero The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The standard enthalpy of formation for an element in its standard state is zero!!!! \[\delta h_{reaction}^o = \sum {\delta. The standard enthalpy of. Standard Enthalpy Change Of Formation Zero.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Change Of Formation Zero 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Elements in their standard state are not formed, they just are. \[\delta h_{reaction}^o = \sum {\delta. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation of any element in its standard state. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT HL Chapter 5 Section 15.1 ppt Focus on Heats of Formation Standard Enthalpy Change Of Formation Zero Elements in their standard state are not formed, they just are. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. \[\delta h_{reaction}^o = \sum {\delta. The standard enthalpy of formation of any element in its standard state is zero by definition. 193. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. To find the δh reaction o, use the formula for the standard enthalpy change of formation: 193 rows in chemistry and thermodynamics, the standard. Standard Enthalpy Change Of Formation Zero.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. Elements in their standard state are not formed, they just. Standard Enthalpy Change Of Formation Zero.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Change Of Formation Zero The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. Elements in their standard state are not formed, they just. Standard Enthalpy Change Of Formation Zero.
From mavink.com
Enthalpy Calculation Standard Enthalpy Change Of Formation Zero To find the δh reaction o, use the formula for the standard enthalpy change of formation: Elements in their standard state are not formed, they just are. \[\delta h_{reaction}^o = \sum {\delta. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation for elements in their most stable form is. Standard Enthalpy Change Of Formation Zero.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Change Of Formation Zero By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of formation. Standard Enthalpy Change Of Formation Zero.
From slideplayer.com
TOPIC 5 ENERGETICS/THERMOCHEMISTRY ppt download Standard Enthalpy Change Of Formation Zero To find the δh reaction o, use the formula for the standard enthalpy change of formation: By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. \[\delta h_{reaction}^o = \sum {\delta. For example, although oxygen can exist as ozone (o 3 ), atomic. Standard Enthalpy Change Of Formation Zero.
From www.slideserve.com
PPT Energy, Enthalpy, and Thermochemistry PowerPoint Presentation Standard Enthalpy Change Of Formation Zero The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation for. Standard Enthalpy Change Of Formation Zero.
From classhirsch.z21.web.core.windows.net
Standard Enthalpy Of Formation Chart Standard Enthalpy Change Of Formation Zero Elements in their standard state are not formed, they just are. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation $\delta h_f^°$ of pure elements is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. \[\delta h_{reaction}^o = \sum {\delta. The standard enthalpy of formation for. Standard Enthalpy Change Of Formation Zero.