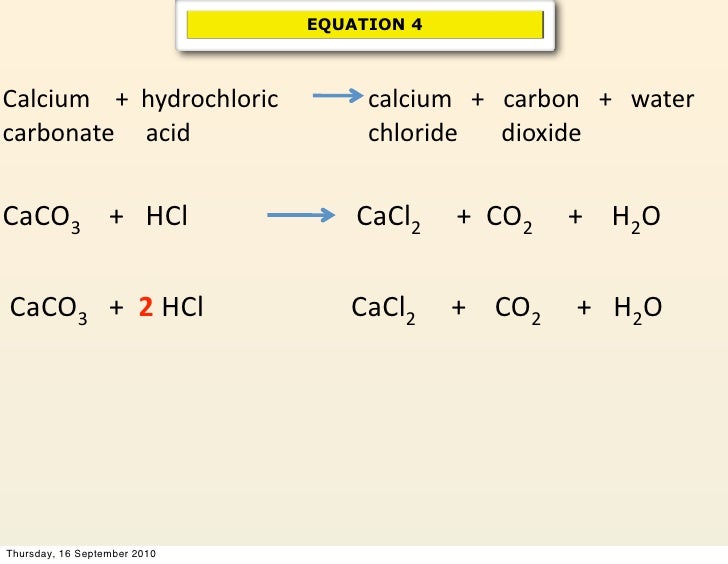
Why Does Calcium React With Hydrochloric Acid . each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. The reaction between calcium and hydrochloric acid. formation of calcium hydroxide. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. These reactions produce hydrogen gas and are. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. Being a group 2 element, calcium is less. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts.
from www.slideshare.net
Danger in contact with water releases flammable gases, see cleapss hazcard hc016. It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. These reactions produce hydrogen gas and are. The reaction between calcium and hydrochloric acid. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. formation of calcium hydroxide. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. Being a group 2 element, calcium is less.
Chemical Reactions
Why Does Calcium React With Hydrochloric Acid It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. These reactions produce hydrogen gas and are. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. formation of calcium hydroxide. The reaction between calcium and hydrochloric acid. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. Being a group 2 element, calcium is less. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute.
From www.transtutors.com
(Get Answer) Consider the reaction between hydrochloric acid and Why Does Calcium React With Hydrochloric Acid These reactions produce hydrogen gas and are. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. Being a group 2 element, calcium is less. The reaction between calcium and hydrochloric acid. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. Danger in contact with water releases flammable. Why Does Calcium React With Hydrochloric Acid.
From niasrfrank.blogspot.com
Calcium Reaction With Dilute Hydrochloric Acid NiasrFrank Why Does Calcium React With Hydrochloric Acid When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. Being a group 2 element, calcium is less. formation of calcium hydroxide. It is safe to carry out the reaction between calcium and hydrochloric acid as long as. Why Does Calcium React With Hydrochloric Acid.
From www.alamy.com
reactivity of different metals with hydrochloric acid calcium Stock Why Does Calcium React With Hydrochloric Acid formation of calcium hydroxide. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. Being a group 2 element, calcium is less. These reactions produce hydrogen gas and are. The reaction between calcium and hydrochloric acid. each metal reacts with dilute hydrochloric acid,. Why Does Calcium React With Hydrochloric Acid.
From weston-kgrimes.blogspot.com
Calcium Reaction With Dilute Hydrochloric Acid Why Does Calcium React With Hydrochloric Acid Danger in contact with water releases flammable gases, see cleapss hazcard hc016. These reactions produce hydrogen gas and are. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. The reaction. Why Does Calcium React With Hydrochloric Acid.
From www.gauthmath.com
Solved Calcium reacts with hydrochloric acid in a single replacement Why Does Calcium React With Hydrochloric Acid It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. . Why Does Calcium React With Hydrochloric Acid.
From linativewise.blogspot.com
Calcium Carbonate and Hydrochloric Acid Why Does Calcium React With Hydrochloric Acid These reactions produce hydrogen gas and are. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. Being a group 2 element, calcium is less. the alkaline earth metals, the second group in the periodic table, have varying degrees. Why Does Calcium React With Hydrochloric Acid.
From www.nagwa.com
Question Video Writing a Net Ionic Equation for the Reaction of Solid Why Does Calcium React With Hydrochloric Acid the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. The reaction between calcium and hydrochloric acid. When calcium (ca2+) is added to dilute 6m hcl solution,. Why Does Calcium React With Hydrochloric Acid.
From skylargokecox.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid Why Does Calcium React With Hydrochloric Acid When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. These reactions produce hydrogen gas and are. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. Being a group 2 element, calcium is less. each metal reacts with dilute hydrochloric. Why Does Calcium React With Hydrochloric Acid.
From www.numerade.com
SOLVED Hydrochloric acid reacts with calcium to form hydrogen and Why Does Calcium React With Hydrochloric Acid Being a group 2 element, calcium is less. These reactions produce hydrogen gas and are. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. It is. Why Does Calcium React With Hydrochloric Acid.
From www.numerade.com
SOLVED When solid calcium hydroxide reacts with hydrochloric acid in Why Does Calcium React With Hydrochloric Acid the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. The reaction between calcium and hydrochloric acid. Being a group 2 element, calcium is less. When calcium. Why Does Calcium React With Hydrochloric Acid.
From keplarllp.com
😀 Calcium hydrochloric acid. PSOW 04. 20190213 Why Does Calcium React With Hydrochloric Acid formation of calcium hydroxide. The reaction between calcium and hydrochloric acid. Being a group 2 element, calcium is less. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. Danger in. Why Does Calcium React With Hydrochloric Acid.
From smallbusinessron.web.fc2.com
calcium and hydrochloric acid Why Does Calcium React With Hydrochloric Acid When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. formation of calcium hydroxide. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally. Why Does Calcium React With Hydrochloric Acid.
From www.vrogue.co
Calcium Hydroxide Reacts With Hydrochloric Acid Balan vrogue.co Why Does Calcium React With Hydrochloric Acid Being a group 2 element, calcium is less. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. formation of calcium hydroxide. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. It is safe to carry out the reaction between calcium. Why Does Calcium React With Hydrochloric Acid.
From kessendrenfinley.blogspot.com
Calcium Chloride and Hydrochloric Acid Balanced Equation KessendrenFinley Why Does Calcium React With Hydrochloric Acid each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. Being a group 2 element, calcium is less. The reaction between calcium and hydrochloric acid. It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. These reactions produce hydrogen gas and. Why Does Calcium React With Hydrochloric Acid.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation ID5408111 Why Does Calcium React With Hydrochloric Acid It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. These reactions produce hydrogen gas and are. formation of calcium hydroxide. Being a group 2 element, calcium is less. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. each metal reacts with dilute. Why Does Calcium React With Hydrochloric Acid.
From www.sciencephoto.com
Calcium reacts with hydrochloric acid, 1 of 3 Stock Image C030/8201 Why Does Calcium React With Hydrochloric Acid When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. Being a group 2 element, calcium is less. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. formation. Why Does Calcium React With Hydrochloric Acid.
From www.numerade.com
SOLVED Calcium carbonate reacts with hydrochloric acid according to Why Does Calcium React With Hydrochloric Acid When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. The reaction between calcium and hydrochloric acid. These reactions produce hydrogen gas and are. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. formation of calcium hydroxide. Danger in contact with water releases flammable gases, see cleapss. Why Does Calcium React With Hydrochloric Acid.
From www.youtube.com
CaCO3 + HCl Calcium Carbonate + Hydrochloric Acid YouTube Why Does Calcium React With Hydrochloric Acid the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. Danger in contact with. Why Does Calcium React With Hydrochloric Acid.
From www.academia.edu
(DOC) What happens if calcium carbonate reacts with hydrochloric acid Why Does Calcium React With Hydrochloric Acid formation of calcium hydroxide. The reaction between calcium and hydrochloric acid. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. the alkaline earth metals,. Why Does Calcium React With Hydrochloric Acid.
From sciencephoto.com
Reaction of calcium in acid Stock Image A500/0757 Science Photo Library Why Does Calcium React With Hydrochloric Acid Danger in contact with water releases flammable gases, see cleapss hazcard hc016. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. Being a group 2 element, calcium is less. These reactions produce hydrogen gas and are. formation of calcium hydroxide. When calcium (ca2+) is added to dilute 6m hcl solution,. Why Does Calcium React With Hydrochloric Acid.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation, free download ID5408111 Why Does Calcium React With Hydrochloric Acid When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. Being a group 2 element, calcium is less. The reaction between calcium and hydrochloric acid. These reactions produce hydrogen gas and are. formation of calcium hydroxide. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all. Why Does Calcium React With Hydrochloric Acid.
From mavink.com
Sodium Metal Reacts With Hydrochloric Acid Why Does Calcium React With Hydrochloric Acid Danger in contact with water releases flammable gases, see cleapss hazcard hc016. These reactions produce hydrogen gas and are. Being a group 2 element, calcium is less. The reaction between calcium and hydrochloric acid. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. When. Why Does Calcium React With Hydrochloric Acid.
From www.youtube.com
The Reaction of Calcium Metal and Hydrochloric Acid YouTube Why Does Calcium React With Hydrochloric Acid each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. These reactions produce hydrogen gas and are. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. It is safe to carry out the reaction between calcium. Why Does Calcium React With Hydrochloric Acid.
From niasrfrank.blogspot.com
Calcium Reaction With Dilute Hydrochloric Acid NiasrFrank Why Does Calcium React With Hydrochloric Acid formation of calcium hydroxide. Being a group 2 element, calcium is less. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. each metal reacts with dilute hydrochloric acid, producing bubbles. Why Does Calcium React With Hydrochloric Acid.
From www.youtube.com
Type of Reaction for Ca + HCl = CaCl2 + H2 YouTube Why Does Calcium React With Hydrochloric Acid the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. These reactions produce hydrogen gas and are. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. It is safe to carry out the reaction between calcium and hydrochloric acid as long as. Why Does Calcium React With Hydrochloric Acid.
From www.vrogue.co
Solved 9 Calcium Carbonate Reacts With Hydrochloric A vrogue.co Why Does Calcium React With Hydrochloric Acid the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. The reaction between calcium and hydrochloric acid. These reactions produce hydrogen gas and are. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. It is safe to carry out the reaction between. Why Does Calcium React With Hydrochloric Acid.
From lexi-bogspotmarsh.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid Why Does Calcium React With Hydrochloric Acid It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. Being a group 2 element, calcium is less. The reaction between calcium and hydrochloric acid. These reactions produce hydrogen gas and are. When calcium (ca2+) is added. Why Does Calcium React With Hydrochloric Acid.
From www.slideshare.net
Chemical Reactions Why Does Calcium React With Hydrochloric Acid These reactions produce hydrogen gas and are. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. formation of calcium hydroxide. It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. the. Why Does Calcium React With Hydrochloric Acid.
From www.gauthmath.com
Solved Calcium reacts with hydrochloric acid in a single replacement Why Does Calcium React With Hydrochloric Acid each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. formation of calcium hydroxide. The reaction between calcium and hydrochloric acid. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. These reactions produce hydrogen gas and are. the alkaline earth metals, the second group in the. Why Does Calcium React With Hydrochloric Acid.
From www.sciencephoto.com
Calcium reacting with hydrochloric acid Stock Image A500/0626 Why Does Calcium React With Hydrochloric Acid These reactions produce hydrogen gas and are. Being a group 2 element, calcium is less. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. formation. Why Does Calcium React With Hydrochloric Acid.
From savanahminwells.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid SavanahminWells Why Does Calcium React With Hydrochloric Acid Being a group 2 element, calcium is less. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. Danger in contact with water releases flammable gases, see cleapss hazcard hc016. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. each. Why Does Calcium React With Hydrochloric Acid.
From mavink.com
Calcium Reacts With Hydrochloric Acid Why Does Calcium React With Hydrochloric Acid Being a group 2 element, calcium is less. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. These reactions produce hydrogen gas and are. formation. Why Does Calcium React With Hydrochloric Acid.
From childhealthpolicy.vumc.org
💄 Calcium carbonate reacts with hydrochloric acid balanced equation Why Does Calcium React With Hydrochloric Acid each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. It is safe to carry out the reaction between calcium and hydrochloric acid as long as the acid is very dilute. When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. These reactions produce hydrogen gas and are. Danger. Why Does Calcium React With Hydrochloric Acid.
From skylargokecox.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid Why Does Calcium React With Hydrochloric Acid Being a group 2 element, calcium is less. the alkaline earth metals, the second group in the periodic table, have varying degrees of activity, but will all generally react with water or. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. When calcium (ca2+) is added to dilute 6m hcl. Why Does Calcium React With Hydrochloric Acid.
From www.sciencephoto.com
Calcium reacts with hydrochloric acid, 3 of 3 Stock Image C030/8203 Why Does Calcium React With Hydrochloric Acid When calcium (ca2+) is added to dilute 6m hcl solution, the ca2+ reacts. Being a group 2 element, calcium is less. each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of. These reactions produce hydrogen gas and are. The reaction between calcium and hydrochloric acid. formation of calcium hydroxide. the. Why Does Calcium React With Hydrochloric Acid.