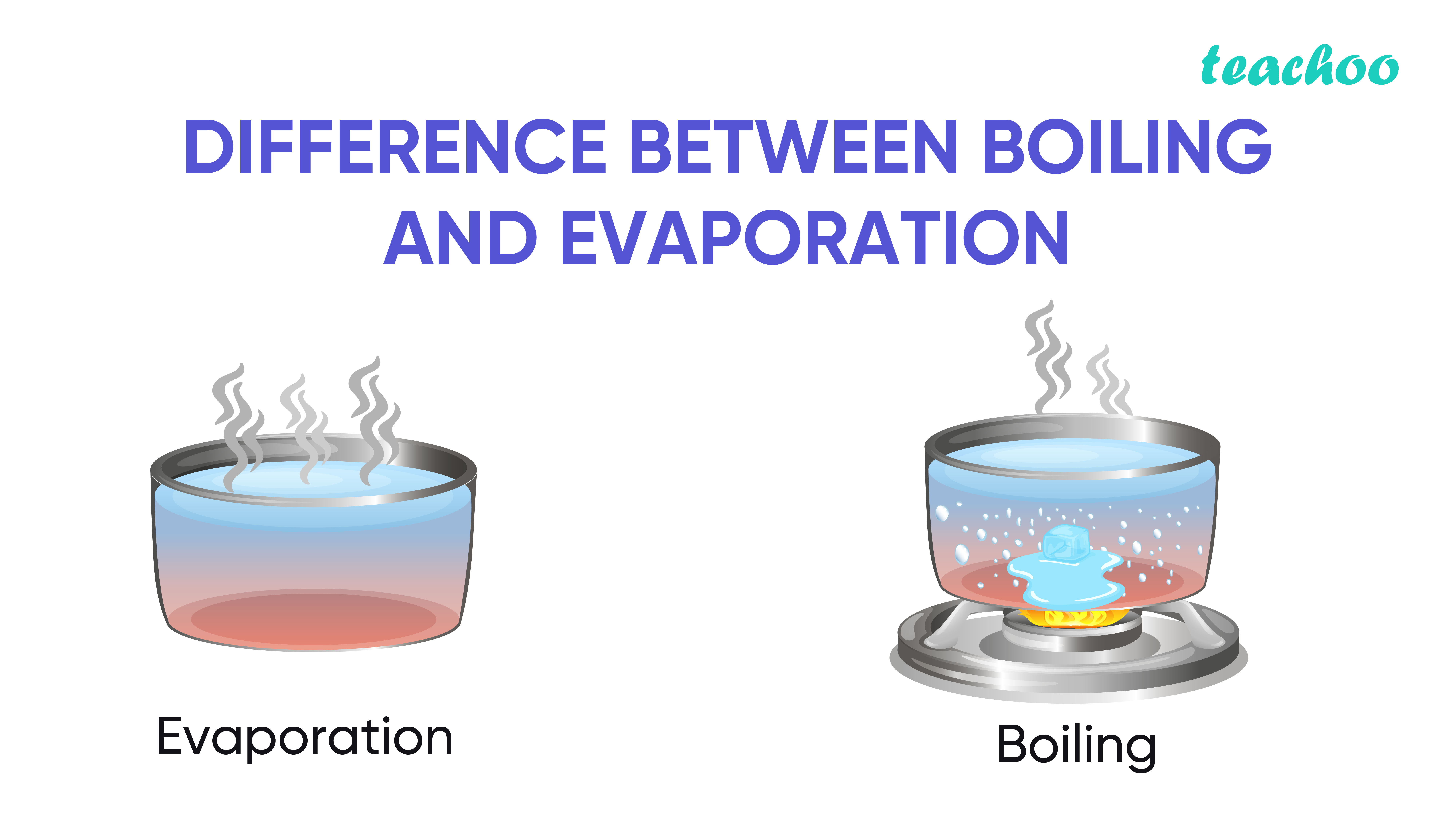
Can Water Evaporate Below Its Boiling Point . water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. On the right, the temperature has been increased until bubbles. When water is heated, it e vaporates. evaporation happens when a liquid substance becomes a gas. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. how does water evaporate without boiling? evaporation occurs when a liquid slowly turns into a gas below its boiling point. Crystallisation is a separation technique used to obtain crystals of a. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling.
from www.teachoo.com
On the right, the temperature has been increased until bubbles. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. how does water evaporate without boiling? evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. evaporation happens when a liquid substance becomes a gas. When water is heated, it e vaporates. evaporation occurs when a liquid slowly turns into a gas below its boiling point. Crystallisation is a separation technique used to obtain crystals of a. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates.
What is the Difference between Evaporation and Boiling? Class 9
Can Water Evaporate Below Its Boiling Point water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. When water is heated, it e vaporates. evaporation happens when a liquid substance becomes a gas. On the right, the temperature has been increased until bubbles. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. Crystallisation is a separation technique used to obtain crystals of a. how does water evaporate without boiling? evaporation occurs when a liquid slowly turns into a gas below its boiling point.
From randall-blogmontgomery.blogspot.com
Is Used to Describe a Liquid That Evaporates Easily Can Water Evaporate Below Its Boiling Point water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. On the right, the temperature has been increased until bubbles. how does water evaporate without boiling? evaporation occurs when a liquid slowly turns into a gas below its boiling point. Crystallisation is a. Can Water Evaporate Below Its Boiling Point.
From cekqjhsz.blob.core.windows.net
Does Salt Evaporate In Boiling Water at Joyce Aybar blog Can Water Evaporate Below Its Boiling Point how does water evaporate without boiling? Crystallisation is a separation technique used to obtain crystals of a. evaporation occurs when a liquid slowly turns into a gas below its boiling point. On the right, the temperature has been increased until bubbles. When water is heated, it e vaporates. evaporation happens when a liquid substance becomes a gas.. Can Water Evaporate Below Its Boiling Point.
From www.numerade.com
Integrated Concepts Heat transfers from your lungs and breathing Can Water Evaporate Below Its Boiling Point Crystallisation is a separation technique used to obtain crystals of a. evaporation happens when a liquid substance becomes a gas. evaporation occurs when a liquid slowly turns into a gas below its boiling point. On the right, the temperature has been increased until bubbles. water easily evaporates at its boiling point (212° f, 100° c) but evaporates. Can Water Evaporate Below Its Boiling Point.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature Can Water Evaporate Below Its Boiling Point evaporation happens when a liquid substance becomes a gas. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. On the right, the temperature has been increased until bubbles. how does water evaporate without boiling? evaporation occurs when a liquid slowly turns. Can Water Evaporate Below Its Boiling Point.
From www.youtube.com
Boiling Point from Heat of Vaporization (Example) YouTube Can Water Evaporate Below Its Boiling Point water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. On the right, the temperature has been increased until bubbles. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. evaporation, process by which. Can Water Evaporate Below Its Boiling Point.
From www.reddit.com
ELI5 why does water evaporate even when it's not at its boiling point Can Water Evaporate Below Its Boiling Point evaporation happens when a liquid substance becomes a gas. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. When water is heated, it e vaporates. how does water evaporate without boiling? Crystallisation is a separation technique used to obtain crystals of a. water easily evaporates at. Can Water Evaporate Below Its Boiling Point.
From www.youtube.com
Evaporation & Boiling Difference YouTube Can Water Evaporate Below Its Boiling Point water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. evaporation happens when a liquid substance becomes a gas. On the right, the temperature has been increased until bubbles. When water is heated, it e vaporates. in the picture on the left, the. Can Water Evaporate Below Its Boiling Point.
From thewaterfiltermarket.com
How Long Does It Take for Water To Evaporate? Water Filter Market Can Water Evaporate Below Its Boiling Point evaporation happens when a liquid substance becomes a gas. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. On the right, the temperature has been increased until bubbles. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its. Can Water Evaporate Below Its Boiling Point.
From www.youtube.com
Boiling water evaporates in 30 Celsius shot at 240fps YouTube Can Water Evaporate Below Its Boiling Point evaporation happens when a liquid substance becomes a gas. how does water evaporate without boiling? On the right, the temperature has been increased until bubbles. evaporation occurs when a liquid slowly turns into a gas below its boiling point. When water is heated, it e vaporates. water easily evaporates at its boiling point (212° f, 100°. Can Water Evaporate Below Its Boiling Point.
From es.scribd.com
How to Evaporate High Boiling Point Solvents Solvent Vacuum Can Water Evaporate Below Its Boiling Point On the right, the temperature has been increased until bubbles. evaporation occurs when a liquid slowly turns into a gas below its boiling point. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. how does water evaporate without boiling? water easily evaporates at its boiling point. Can Water Evaporate Below Its Boiling Point.
From www.youtube.com
How does Water Evaporate below its Boiling Point? CBSE Class 9 & 10 Can Water Evaporate Below Its Boiling Point evaporation occurs when a liquid slowly turns into a gas below its boiling point. how does water evaporate without boiling? On the right, the temperature has been increased until bubbles. Crystallisation is a separation technique used to obtain crystals of a. evaporation, process by which an element or compound transitions from its liquid state to its gaseous. Can Water Evaporate Below Its Boiling Point.
From cefnhtgk.blob.core.windows.net
What Temperature Does Water Boil With Salt at Mary Everman blog Can Water Evaporate Below Its Boiling Point how does water evaporate without boiling? evaporation occurs when a liquid slowly turns into a gas below its boiling point. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. in the picture on the left, the liquid is below its boiling. Can Water Evaporate Below Its Boiling Point.
From cerismrq.blob.core.windows.net
Evaporation And Heat at Brian West blog Can Water Evaporate Below Its Boiling Point On the right, the temperature has been increased until bubbles. how does water evaporate without boiling? in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. evaporation happens when a liquid substance becomes a gas. evaporation, process by which an element or compound transitions from its liquid. Can Water Evaporate Below Its Boiling Point.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Can Water Evaporate Below Its Boiling Point evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. On the right, the temperature has been increased until bubbles. evaporation occurs when a liquid slowly turns into. Can Water Evaporate Below Its Boiling Point.
From www.numerade.com
⏩SOLVEDVaporization at the normal boiling point (Tvap ) of a… Numerade Can Water Evaporate Below Its Boiling Point evaporation occurs when a liquid slowly turns into a gas below its boiling point. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. how does water evaporate without boiling? Crystallisation is a separation technique used to obtain crystals of a. in the picture on the. Can Water Evaporate Below Its Boiling Point.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Can Water Evaporate Below Its Boiling Point water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. evaporation, process by which an element or compound transitions from its liquid state to. Can Water Evaporate Below Its Boiling Point.
From ar.inspiredpencil.com
Boiling Point Of Water Examples Can Water Evaporate Below Its Boiling Point in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. evaporation occurs when a liquid slowly turns into a gas below its boiling point.. Can Water Evaporate Below Its Boiling Point.
From telgurus.co.uk
How do I know if an enthalpy change should be positive or negative? Can Water Evaporate Below Its Boiling Point When water is heated, it e vaporates. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. evaporation occurs when a liquid slowly turns into a gas below. Can Water Evaporate Below Its Boiling Point.
From www.youtube.com
ALEKS Using Heat of Fusion or Vaporization to Find the Heat Needed to Can Water Evaporate Below Its Boiling Point When water is heated, it e vaporates. On the right, the temperature has been increased until bubbles. evaporation happens when a liquid substance becomes a gas. Crystallisation is a separation technique used to obtain crystals of a. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because. Can Water Evaporate Below Its Boiling Point.
From circuitdiagramlows.z22.web.core.windows.net
Phase Change Diagram For Water Can Water Evaporate Below Its Boiling Point water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. On the right, the temperature has been increased until bubbles. how does water evaporate without boiling? evaporation happens when a liquid substance becomes a gas. evaporation, process by which an element or. Can Water Evaporate Below Its Boiling Point.
From www.numerade.com
VIDEO solution Molar heat of vaporization is described as Heat Can Water Evaporate Below Its Boiling Point evaporation happens when a liquid substance becomes a gas. how does water evaporate without boiling? in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. On the right, the temperature has been increased until bubbles. evaporation occurs when a liquid slowly turns into a gas below its. Can Water Evaporate Below Its Boiling Point.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Can Water Evaporate Below Its Boiling Point how does water evaporate without boiling? evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. evaporation happens when a liquid substance becomes a gas. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because. Can Water Evaporate Below Its Boiling Point.
From edu.rsc.org
Evaporation explained Infographic RSC Education Can Water Evaporate Below Its Boiling Point Crystallisation is a separation technique used to obtain crystals of a. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. evaporation occurs when a liquid slowly turns into a gas below its boiling point. how does water evaporate without boiling? On the right, the temperature has been. Can Water Evaporate Below Its Boiling Point.
From chem.libretexts.org
10.4 Properties of Liquids Chemistry LibreTexts Can Water Evaporate Below Its Boiling Point evaporation happens when a liquid substance becomes a gas. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. When water is heated,. Can Water Evaporate Below Its Boiling Point.
From www.numerade.com
SOLVED The essential oils of plants often have a characteristic smell Can Water Evaporate Below Its Boiling Point When water is heated, it e vaporates. evaporation happens when a liquid substance becomes a gas. Crystallisation is a separation technique used to obtain crystals of a. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. evaporation, process by which an element or compound transitions from its. Can Water Evaporate Below Its Boiling Point.
From www.numerade.com
SOLVED CHAPTER 11 HOMEWORK ASSIGNMENT Determine the kinds of Can Water Evaporate Below Its Boiling Point Crystallisation is a separation technique used to obtain crystals of a. When water is heated, it e vaporates. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. evaporation happens when a liquid substance becomes a gas. On the right, the temperature has been increased until bubbles. . Can Water Evaporate Below Its Boiling Point.
From brainly.com
Calculate the amount of heat released when 27.0 g H2O is cooled from a Can Water Evaporate Below Its Boiling Point evaporation happens when a liquid substance becomes a gas. When water is heated, it e vaporates. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. evaporation. Can Water Evaporate Below Its Boiling Point.
From slideplayer.com
MATTER. ppt download Can Water Evaporate Below Its Boiling Point water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. evaporation occurs when a liquid slowly turns into a gas below its boiling point. Crystallisation is a separation technique used to obtain crystals of a. how does water evaporate without boiling? On the. Can Water Evaporate Below Its Boiling Point.
From bigtimekitchen.com
Does Water Evaporate Faster With Or Without A Lid? Can Water Evaporate Below Its Boiling Point water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. Crystallisation is a separation technique used to obtain crystals of a. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. When water is. Can Water Evaporate Below Its Boiling Point.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Can Water Evaporate Below Its Boiling Point When water is heated, it e vaporates. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. evaporation happens when a liquid substance becomes a gas. how does water evaporate without boiling? On the right, the temperature has been increased until bubbles. evaporation, process by which an. Can Water Evaporate Below Its Boiling Point.
From thehobbykraze.com
Can Snow Evaporate Instead Of Melt? (How It Disappears Without A Trace Can Water Evaporate Below Its Boiling Point evaporation happens when a liquid substance becomes a gas. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. Crystallisation is a separation technique used to obtain crystals of a. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below. Can Water Evaporate Below Its Boiling Point.
From www.scribd.com
How Does Water Evaporate Below Its Boiling Point Quora PDF Can Water Evaporate Below Its Boiling Point On the right, the temperature has been increased until bubbles. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat. how does water evaporate without boiling? evaporation happens when a liquid substance becomes a gas. Crystallisation is a separation technique used to obtain. Can Water Evaporate Below Its Boiling Point.
From nittygrittyscience.com
Section 5 Changes in States of Matter Nitty Gritty Science Can Water Evaporate Below Its Boiling Point how does water evaporate without boiling? When water is heated, it e vaporates. Crystallisation is a separation technique used to obtain crystals of a. On the right, the temperature has been increased until bubbles. evaporation happens when a liquid substance becomes a gas. evaporation, process by which an element or compound transitions from its liquid state to. Can Water Evaporate Below Its Boiling Point.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Can Water Evaporate Below Its Boiling Point evaporation occurs when a liquid slowly turns into a gas below its boiling point. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. When water is heated,. Can Water Evaporate Below Its Boiling Point.
From www.numerade.com
SOLVED '3. Calculate the mass of water (in g) that can be vaporized at Can Water Evaporate Below Its Boiling Point evaporation occurs when a liquid slowly turns into a gas below its boiling point. in the picture on the left, the liquid is below its boiling point, yet some of the liquid evaporates. Crystallisation is a separation technique used to obtain crystals of a. On the right, the temperature has been increased until bubbles. water easily evaporates. Can Water Evaporate Below Its Boiling Point.