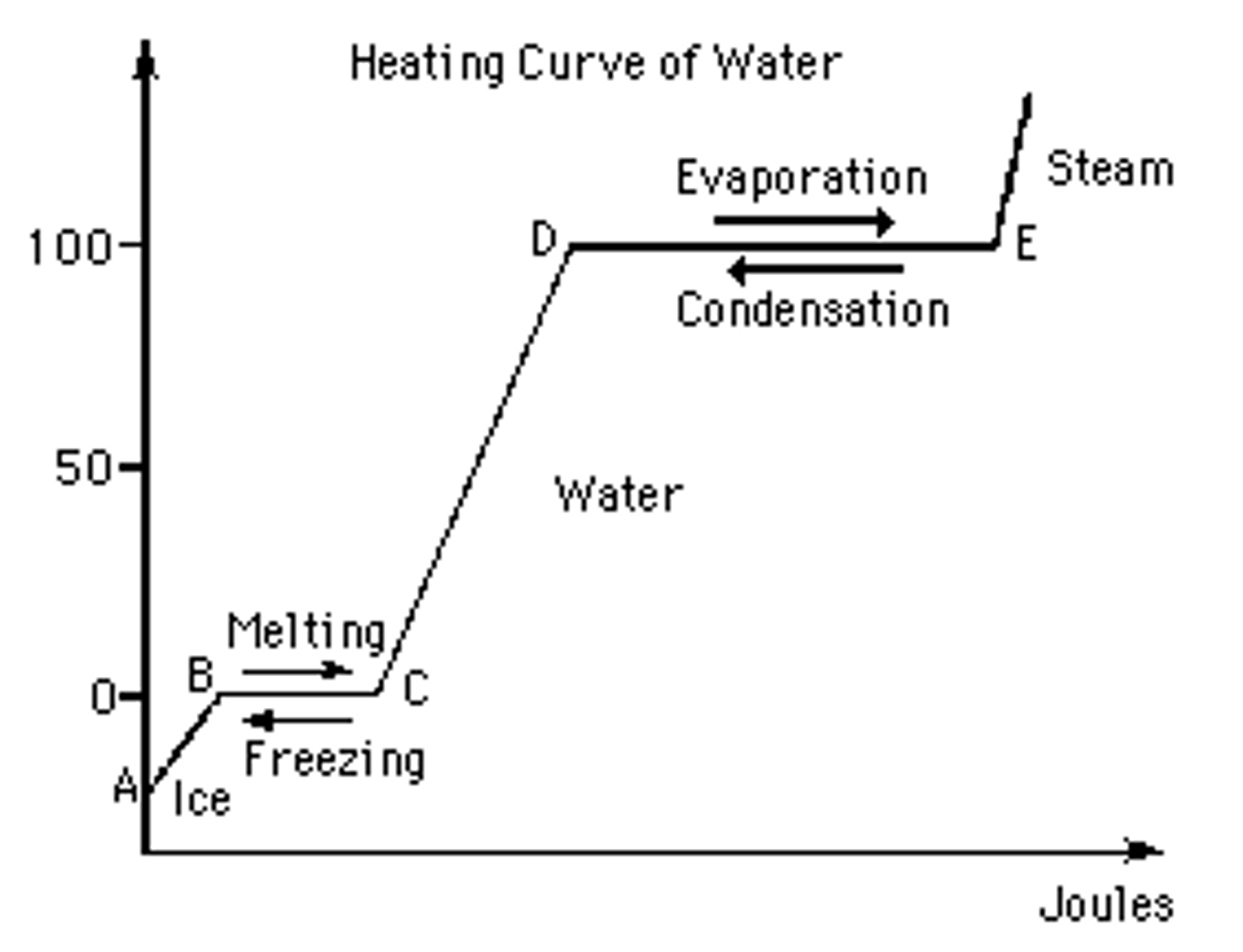
Heating Curve Fusion . As heat is added, the temperature of the ice increases linearly with time. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant pressure. The sample is initially ice at 1 atm and −23°c; A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). This exercise explores the changes that occur to a. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. (in this context, fusion is another word for melting.) the units for δhfus. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Plateaus in the curve (regions of constant.
from exobuhknu.blob.core.windows.net
Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. This exercise explores the changes that occur to a. Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant pressure. (in this context, fusion is another word for melting.) the units for δhfus. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Plateaus in the curve (regions of constant. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. As heat is added, the temperature of the ice increases linearly with time. A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat.
Heating Curve Of Substance X at Nina Edwards blog
Heating Curve Fusion As heat is added, the temperature of the ice increases linearly with time. A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). As heat is added, the temperature of the ice increases linearly with time. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant pressure. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Plateaus in the curve (regions of constant. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. This exercise explores the changes that occur to a. (in this context, fusion is another word for melting.) the units for δhfus. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. The sample is initially ice at 1 atm and −23°c;
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heating Curve Fusion The sample is initially ice at 1 atm and −23°c; Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant. Heating Curve Fusion.
From chem.libretexts.org
5.5.1 Heating Curves and Phase Changes (Problems) Chemistry LibreTexts Heating Curve Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. (in this context, fusion is another word for melting.) the units for δhfus. This exercise explores the changes that occur to a. Heat of fusion, δhfus, is the heat energy. Heating Curve Fusion.
From www.slideserve.com
PPT The Heating Curve PowerPoint Presentation, free download ID5070642 Heating Curve Fusion Boiling or vaporization is an example of a phase change from the liquid to the gas phase. The sample is initially ice at 1 atm and −23°c; (in this context, fusion is another word for melting.) the units for δhfus. A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of. Heating Curve Fusion.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Heating Curve Fusion Plateaus in the curve (regions of constant. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The sample. Heating Curve Fusion.
From study.com
What are Heating and Cooling Curves? Video & Lesson Transcript Heating Curve Fusion The sample is initially ice at 1 atm and −23°c; A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Boiling or vaporization is an example of a phase change from the liquid to the gas phase. A typical heating curve for a substance depicts changes in temperature that result. Heating Curve Fusion.
From worksheetlistddt.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating Curve Fusion Plateaus in the curve (regions of constant. Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant pressure. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. (in this context, fusion is another word for melting.) the units for δhfus. As heat is added, the. Heating Curve Fusion.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Curve Fusion As heat is added, the temperature of the ice increases linearly with time. A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. (in this context, fusion is another word for melting.) the units for δhfus.. Heating Curve Fusion.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curve Fusion (in this context, fusion is another word for melting.) the units for δhfus. This exercise explores the changes that occur to a. A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Boiling or vaporization. Heating Curve Fusion.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heating Curve Fusion Plateaus in the curve (regions of constant. Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant pressure. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. A typical heating curve for a substance depicts changes in temperature that result as the. Heating Curve Fusion.
From curiophysics.com
Heating Curve » Curio Physics Heating Curve Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are. Heating Curve Fusion.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heating Curve Fusion Plateaus in the curve (regions of constant. A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. A plot of the temperature versus the amount of heat added is known as a heating curve (see. Heating Curve Fusion.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Heating Curve Fusion As heat is added, the temperature of the ice increases linearly with time. The sample is initially ice at 1 atm and −23°c; A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. (in this. Heating Curve Fusion.
From www.chegg.com
Solved Q5 Heating Curve 2 Points Consider the following Heating Curve Fusion As heat is added, the temperature of the ice increases linearly with time. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Plateaus in the curve (regions of constant. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. A typical heating curve for a substance depicts changes. Heating Curve Fusion.
From exobuhknu.blob.core.windows.net
Heating Curve Of Substance X at Nina Edwards blog Heating Curve Fusion As heat is added, the temperature of the ice increases linearly with time. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Plateaus in the. Heating Curve Fusion.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. This exercise explores the changes that occur to a. As heat is added, the temperature of the ice increases linearly with time. Plateaus in the curve (regions of constant. A. Heating Curve Fusion.
From www.slideserve.com
PPT Exothermic and Endothermic Processes PowerPoint Presentation Heating Curve Fusion This exercise explores the changes that occur to a. A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Plateaus in the curve (regions of constant. (in this context, fusion is another word for melting.) the units for δhfus. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus. Heating Curve Fusion.
From slideplayer.com
Heating and Cooling Curves ppt download Heating Curve Fusion Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. As heat is added, the temperature of the ice increases linearly with time. (in this context, fusion is another word for melting.) the units for δhfus. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. This exercise explores. Heating Curve Fusion.
From www.slideserve.com
PPT Heating Curves and Specific Heat PowerPoint Presentation, free Heating Curve Fusion Plateaus in the curve (regions of constant. The sample is initially ice at 1 atm and −23°c; As heat is added, the temperature of the ice increases linearly with time. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time,. Heating Curve Fusion.
From www.chegg.com
Solved Refer to the heating curve below. On the basis Heating Curve Fusion (in this context, fusion is another word for melting.) the units for δhfus. Plateaus in the curve (regions of constant. The sample is initially ice at 1 atm and −23°c; Boiling or vaporization is an example of a phase change from the liquid to the gas phase. This exercise explores the changes that occur to a. Figure \(\pageindex{3}\) shows a. Heating Curve Fusion.
From slideplayer.com
Heating Curves and Phase Diagrams ppt download Heating Curve Fusion Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant pressure. As heat is added, the temperature of the ice increases linearly with time. (in this context, fusion is another word for melting.) the units for. Heating Curve Fusion.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating Curve Fusion Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. This exercise explores the changes that occur to a. A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g. Heating Curve Fusion.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Curve Fusion Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant pressure. Plateaus in the curve (regions of constant. A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. This exercise explores the changes that occur to a. Figure \(\pageindex{3}\). Heating Curve Fusion.
From www.slideserve.com
PPT The Heating Curve PowerPoint Presentation, free download ID5070642 Heating Curve Fusion Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. (in this context, fusion is another word for melting.) the units for δhfus. Boiling or vaporization is an example of a phase change from the. Heating Curve Fusion.
From chem.libretexts.org
5.5 Heating Curves and Phase Changes Chemistry LibreTexts Heating Curve Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). This exercise explores the changes that occur to a. Fusion,. Heating Curve Fusion.
From spmphysics.onlinetuition.com.my
The Heating Curve SPM Physics Form 4/Form 5 Revision Notes Heating Curve Fusion Boiling or vaporization is an example of a phase change from the liquid to the gas phase. Plateaus in the curve (regions of constant. Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant pressure. As heat is added, the temperature of the ice increases linearly with time. Figure \(\pageindex{3}\). Heating Curve Fusion.
From ch301.cm.utexas.edu
heating curve Heating Curve Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. This exercise explores the changes that occur to a. As. Heating Curve Fusion.
From philschatz.com
Phase Change and Latent Heat · Physics Heating Curve Fusion Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant pressure. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. As heat is added, the temperature of the ice increases linearly with time. Boiling or vaporization is an example of a phase change from the. Heating Curve Fusion.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Heating Curve Fusion A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Boiling or vaporization is an example of a phase change from the liquid to the gas phase. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze. Heating Curve Fusion.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 Heating Curve Fusion Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Plateaus in the curve (regions of constant. The sample is initially ice at 1 atm and −23°c; Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. This exercise explores the changes that occur to a.. Heating Curve Fusion.
From www.youtube.com
Heating Curve Practice 3 Step with Phase Change Fusion YouTube Heating Curve Fusion Plateaus in the curve (regions of constant. A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. (in this context, fusion is another word for melting.) the units for. Heating Curve Fusion.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Fusion Heat of fusion, δhfus, is the heat energy necessary to convert a given amount of solid to liquid at constant pressure. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed. Heating Curve Fusion.
From www.youtube.com
Heating Curves Tutorial How to Calculate enthalpy changes in Heating Heating Curve Fusion A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The sample is initially ice. Heating Curve Fusion.
From www.chegg.com
Solved Using the following heating curve, what line Heating Curve Fusion Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. This exercise explores the changes that. Heating Curve Fusion.
From www.slideserve.com
PPT Heating Curve at Constant Pressure PowerPoint Presentation, free Heating Curve Fusion (in this context, fusion is another word for melting.) the units for δhfus. The sample is initially ice at 1 atm and −23°c; Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. As heat is added, the temperature of the ice increases linearly with time. A plot of the. Heating Curve Fusion.
From courses.lumenlearning.com
Phase Transitions Chemistry Atoms First Heating Curve Fusion A plot of the temperature versus the amount of heat added is known as a heating curve (see figure 10.18). Plateaus in the curve (regions of constant. The sample is initially ice at 1 atm and −23°c; Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze. Heating Curve Fusion.