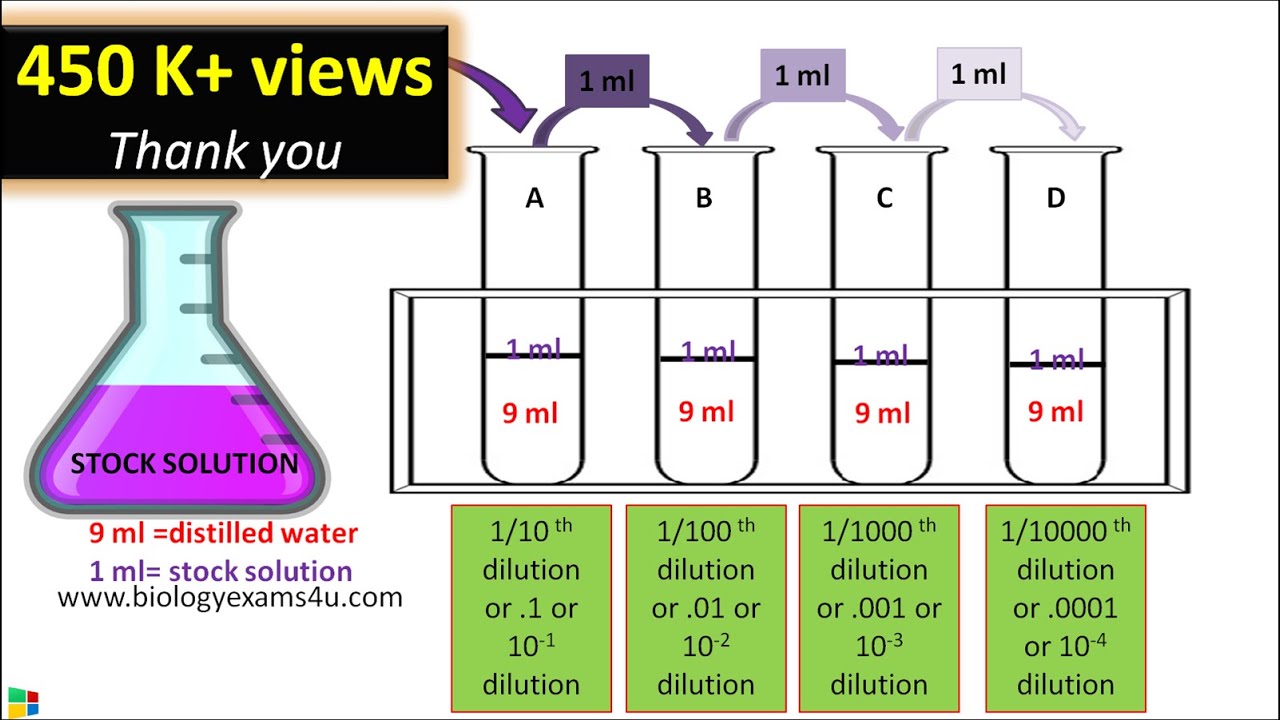
Dilution In Example . dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Concentration is the removal of solvent, which. There are many ways of expressing concentration. Explanations and examples of common methods. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute.
from www.youtube.com
dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. Concentration is the removal of solvent, which. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. There are many ways of expressing concentration. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute.
Serial Dilution Method Protocol Step Wise Explanation YouTube
Dilution In Example dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Concentration is the removal of solvent, which. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. There are many ways of expressing concentration. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Explanations and examples of common methods. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the.
From www.slideserve.com
PPT Dilutions PowerPoint Presentation, free download ID226520 Dilution In Example There are many ways of expressing concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. dilution is the process of “lowering the concentration of a. Dilution In Example.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilution In Example dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Explanations and examples of common methods. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution is the process of “lowering the concentration of a solute. Dilution In Example.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution In Example dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration. Concentration is the removal of solvent, which. Explanations and examples of common methods. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution).. Dilution In Example.
From www.medicine.mcgill.ca
Serial Dilutions Dilution In Example Concentration is the removal of solvent, which. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Explanations and examples of common methods. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. dilution. Dilution In Example.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Dilution In Example Explanations and examples of common methods. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Concentration is the removal of solvent, which. There are many ways of expressing concentration.. Dilution In Example.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution In Example There are many ways of expressing concentration. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise mass of solute in a precise volume of. Dilution In Example.
From www.showme.com
Dilution calculation Science, Chemicalreactions, Biology ShowMe Dilution In Example dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Explanations and examples of common methods. Concentration is the removal of solvent, which. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilution is a process. Dilution In Example.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution In Example Explanations and examples of common methods. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). dilution is the process of making. Dilution In Example.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution In Example a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Explanations and examples of common methods. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of “lowering the concentration of a solute in a. Dilution In Example.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution In Example Explanations and examples of common methods. Concentration is the removal of solvent, which. There are many ways of expressing concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution).. Dilution In Example.
From giokewoed.blob.core.windows.net
How Does Dilution Work Chemistry at Margaret Gamble blog Dilution In Example Concentration is the removal of solvent, which. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. dilution is the addition of solvent, which. Dilution In Example.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution In Example dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Explanations and examples of common methods. dilution is the process of making a solution or scientific sample less concentrated. Dilution In Example.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution In Example a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. There are many ways of expressing concentration. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. dilution is the process of “lowering the concentration of a. Dilution In Example.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution In Example a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. dilution is the addition of solvent, which decreases the concentration of the. Dilution In Example.
From carlosgokeowen.blogspot.com
What is Dilution Dilution In Example There are many ways of expressing concentration. Explanations and examples of common methods. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Concentration is the removal of solvent, which. a solution containing a. Dilution In Example.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution In Example Explanations and examples of common methods. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Concentration is the removal of solvent, which. . Dilution In Example.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution In Example dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. There are many ways of expressing concentration. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilution is a process. Dilution In Example.
From www.slideserve.com
PPT Dilutions PowerPoint Presentation, free download ID226520 Dilution In Example a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). dilution is the addition of solvent, which decreases the concentration of the. Dilution In Example.
From www.slideshare.net
Serial dilution Dilution In Example dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. There are many ways of expressing concentration. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a dilution is a process where the concentration of a. Dilution In Example.
From fyolbkacc.blob.core.windows.net
Dilution Definition In Chemistry at Pamela Wagner blog Dilution In Example a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. dilution is the process of making. Dilution In Example.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution In Example dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. a dilution is a process where the concentration of a solution is lowered by adding solvent to. Dilution In Example.
From www.slideserve.com
PPT Dilutions PowerPoint Presentation, free download ID9606317 Dilution In Example Explanations and examples of common methods. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). There are many ways of expressing concentration. Concentration is. Dilution In Example.
From www.slideserve.com
PPT Dilutions PowerPoint Presentation, free download ID226520 Dilution In Example Concentration is the removal of solvent, which. There are many ways of expressing concentration. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Explanations and examples of common methods. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the.. Dilution In Example.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution In Example Explanations and examples of common methods. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). dilution is the process of “lowering. Dilution In Example.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution In Example dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. There are many ways of expressing concentration. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Explanations and examples of common methods. a dilution is a process. Dilution In Example.
From facts.net
13 Surprising Facts About Dilution Dilution In Example a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Concentration is the removal of solvent, which. dilution is the process of. Dilution In Example.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution In Example Explanations and examples of common methods. Concentration is the removal of solvent, which. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise. Dilution In Example.
From gioartpaa.blob.core.windows.net
What Are Dilutions Chemistry at Julian Smith blog Dilution In Example a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Explanations and examples of common methods. dilution is the process of “lowering. Dilution In Example.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilution In Example There are many ways of expressing concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Concentration is the removal of solvent, which. dilution is the process. Dilution In Example.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer Dilution In Example dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Concentration is the removal of solvent, which. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilution is a process where the concentration of a solution. Dilution In Example.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution In Example Concentration is the removal of solvent, which. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). There are many ways of expressing concentration. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. Dilution In Example.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution In Example dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Explanations and examples of common methods. dilution is the process of “lowering the concentration of a solute in a. Dilution In Example.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution In Example a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Explanations and examples of common methods. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution is the process of making. Dilution In Example.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution In Example dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). There are. Dilution In Example.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution In Example Explanations and examples of common methods. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. a dilution is a process where the concentration of a solution is lowered. Dilution In Example.