What Is The Valence Shell Configuration Of Arsenic . arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. The total number of electrons in a valence shell is called valence electrons. arsenic number of valence electrons. The dots are placed on the four sides of the symbol, with one dot on each side and a fifth dot placed between two sides. Ground state electron configuration for arsenic. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. Therefore, the valence electrons of arsenic are five. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. The electron configuration shows that the last shell of arsenic has five electrons. the last shell after the electron configuration is called the valence shell. In the case of arsenic, there are 5 electrons in the outer or fourth shell. arsenic has 5 valence electrons. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the. arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. These electrons are represented by dots surrounding the chemical symbol as in the diagram.
from www.youtube.com
arsenic, located in group 15 of the periodic table, has five valence electrons. arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. arsenic number of valence electrons. These electrons are represented by dots surrounding the chemical symbol as in the diagram. the last shell after the electron configuration is called the valence shell. arsenic has 5 valence electrons. The total number of electrons in a valence shell is called valence electrons. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3.
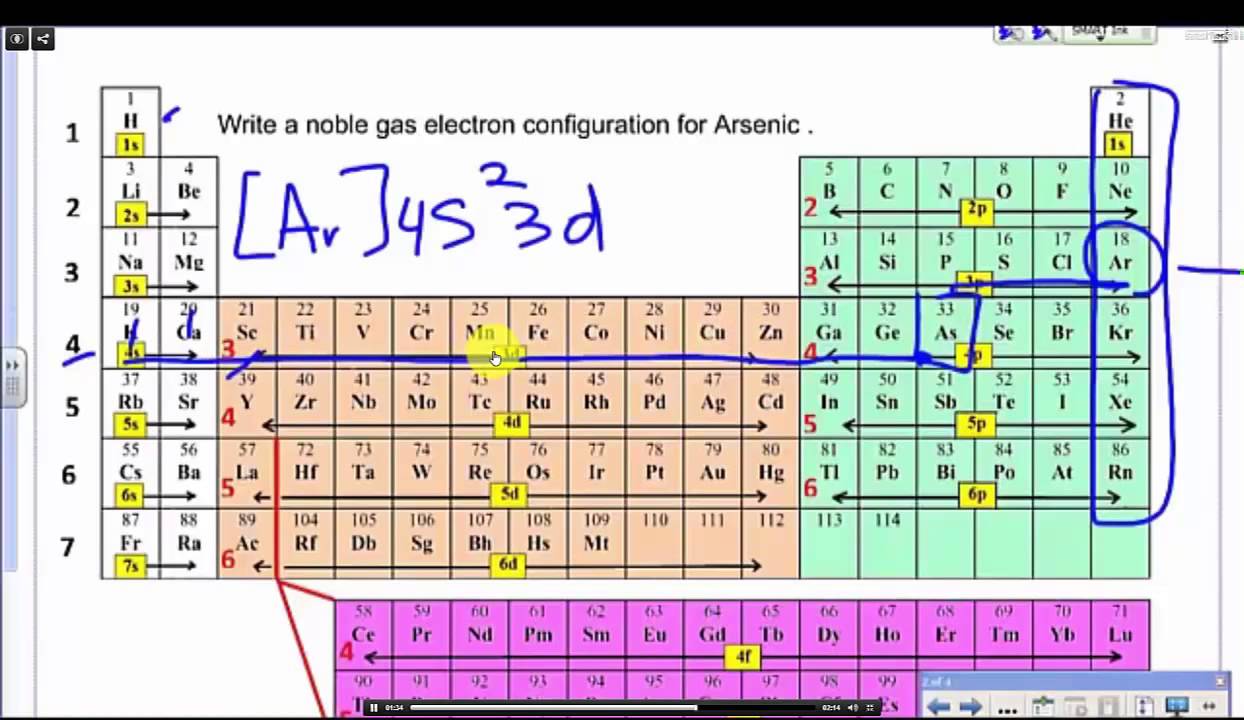
Write a noble gas or shorthand electron configuration arsenic YouTube
What Is The Valence Shell Configuration Of Arsenic Ground state electron configuration for arsenic. These electrons are represented by dots surrounding the chemical symbol as in the diagram. The electron configuration shows that the last shell of arsenic has five electrons. arsenic, located in group 15 of the periodic table, has five valence electrons. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. In the case of arsenic, there are 5 electrons in the outer or fourth shell. Therefore, the valence electrons of arsenic are five. The total number of electrons in a valence shell is called valence electrons. The dots are placed on the four sides of the symbol, with one dot on each side and a fifth dot placed between two sides. arsenic number of valence electrons. the last shell after the electron configuration is called the valence shell. arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. arsenic has 5 valence electrons. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the. Ground state electron configuration for arsenic.
From www.youtube.com
How to Find the Valence Electrons for Arsenic (As) YouTube What Is The Valence Shell Configuration Of Arsenic arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. Therefore, the valence electrons of arsenic are five. arsenic has 5 valence electrons. In the case of arsenic, there are 5 electrons in the outer or fourth shell. arsenic number of valence electrons. Ground state. What Is The Valence Shell Configuration Of Arsenic.
From stock.adobe.com
Vetor de Arsenic atomic structure has atomic number, atomic mass What Is The Valence Shell Configuration Of Arsenic It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the. These electrons are represented by dots surrounding the chemical symbol as. What Is The Valence Shell Configuration Of Arsenic.
From animalia-life.club
Electron Configuration Of Arsenic What Is The Valence Shell Configuration Of Arsenic The total number of electrons in a valence shell is called valence electrons. These electrons are represented by dots surrounding the chemical symbol as in the diagram. The electron configuration shows that the last shell of arsenic has five electrons. Ground state electron configuration for arsenic. arsenic has 5 valence electrons. The dots are placed on the four sides. What Is The Valence Shell Configuration Of Arsenic.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is The Valence Shell Configuration Of Arsenic Therefore, the valence electrons of arsenic are five. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. In the case of arsenic, there are 5 electrons in the outer or fourth shell. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. arsenic number of valence electrons.. What Is The Valence Shell Configuration Of Arsenic.
From www.alamy.com
Periodic Table of the Elements, Shell Structure of Arsenic As What Is The Valence Shell Configuration Of Arsenic The electron configuration shows that the last shell of arsenic has five electrons. arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. arsenic has 5 valence electrons. Therefore, the valence electrons of arsenic are five. The total number of electrons in a valence shell is. What Is The Valence Shell Configuration Of Arsenic.
From valenceelectrons.com
Electron Configuration for Arsenic (As, As3 ion) What Is The Valence Shell Configuration Of Arsenic arsenic number of valence electrons. arsenic, located in group 15 of the periodic table, has five valence electrons. The dots are placed on the four sides of the symbol, with one dot on each side and a fifth dot placed between two sides. The electron configuration shows that the last shell of arsenic has five electrons. Therefore, the. What Is The Valence Shell Configuration Of Arsenic.
From www.alamy.com
Arsenic (As). Diagram of the nuclear composition, electron What Is The Valence Shell Configuration Of Arsenic Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. The dots are placed on the four sides of the symbol, with one dot on each side and a fifth dot placed between two sides. arsenic has 5 valence electrons. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10. What Is The Valence Shell Configuration Of Arsenic.
From ar.inspiredpencil.com
Electron Configuration Of Arsenic Shorthand What Is The Valence Shell Configuration Of Arsenic arsenic number of valence electrons. Therefore, the valence electrons of arsenic are five. The dots are placed on the four sides of the symbol, with one dot on each side and a fifth dot placed between two sides. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the. arsenic, located in. What Is The Valence Shell Configuration Of Arsenic.
From mavink.com
Periodic Table Valence Electrons What Is The Valence Shell Configuration Of Arsenic arsenic has 5 valence electrons. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. In the case of arsenic, there are 5 electrons in the outer or fourth shell. Therefore, the valence electrons of arsenic are. What Is The Valence Shell Configuration Of Arsenic.
From animalia-life.club
Arsenic Bohr Model What Is The Valence Shell Configuration Of Arsenic Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. The total number of electrons in a valence shell is called valence electrons. Therefore, the valence electrons of arsenic are five. Ground state electron configuration for arsenic. arsenic number of valence electrons. arsenic is a chemical element of the. What Is The Valence Shell Configuration Of Arsenic.
From www.youtube.com
Write Electron Configuration for Arsenic YouTube What Is The Valence Shell Configuration Of Arsenic It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. The total number of electrons in a valence shell is called valence electrons. arsenic, located in group 15 of the periodic table, has five valence electrons. arsenic number of valence electrons. Valence electrons are the number of electrons that are present at the outermost shell of. What Is The Valence Shell Configuration Of Arsenic.
From periodictable.me
Arsenic Electron Configuration (As) with Orbital Diagram What Is The Valence Shell Configuration Of Arsenic These electrons are represented by dots surrounding the chemical symbol as in the diagram. In the case of arsenic, there are 5 electrons in the outer or fourth shell. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. arsenic number of valence electrons. The dots are placed on the four sides of the symbol, with one. What Is The Valence Shell Configuration Of Arsenic.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is The Valence Shell Configuration Of Arsenic arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. These electrons are represented by dots surrounding the chemical symbol as in the diagram. arsenic, located in group 15 of the periodic table, has five valence electrons. arsenic number of valence electrons. Therefore, the valence electrons of arsenic. What Is The Valence Shell Configuration Of Arsenic.
From commons.wikimedia.org
FileElectron shell 033 arsenic.png What Is The Valence Shell Configuration Of Arsenic The electron configuration shows that the last shell of arsenic has five electrons. The dots are placed on the four sides of the symbol, with one dot on each side and a fifth dot placed between two sides. arsenic has 5 valence electrons. arsenic number of valence electrons. arsenic is a chemical element of the periodic table. What Is The Valence Shell Configuration Of Arsenic.
From www.reference.com
What Is the Electron Configuration of Arsenic? What Is The Valence Shell Configuration Of Arsenic In the case of arsenic, there are 5 electrons in the outer or fourth shell. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. It's electron configuration. What Is The Valence Shell Configuration Of Arsenic.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts What Is The Valence Shell Configuration Of Arsenic arsenic, located in group 15 of the periodic table, has five valence electrons. arsenic has 5 valence electrons. In the case of arsenic, there are 5 electrons in the outer or fourth shell. arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. Valence electrons are the number. What Is The Valence Shell Configuration Of Arsenic.
From material-properties.org
Arsenic Periodic Table and Atomic Properties What Is The Valence Shell Configuration Of Arsenic the last shell after the electron configuration is called the valence shell. These electrons are represented by dots surrounding the chemical symbol as in the diagram. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. arsenic. What Is The Valence Shell Configuration Of Arsenic.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary What Is The Valence Shell Configuration Of Arsenic These electrons are represented by dots surrounding the chemical symbol as in the diagram. the last shell after the electron configuration is called the valence shell. The dots are placed on the four sides of the symbol, with one dot on each side and a fifth dot placed between two sides. Ground state electron configuration for arsenic. arsenic. What Is The Valence Shell Configuration Of Arsenic.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry What Is The Valence Shell Configuration Of Arsenic The dots are placed on the four sides of the symbol, with one dot on each side and a fifth dot placed between two sides. Ground state electron configuration for arsenic. These electrons are represented by dots surrounding the chemical symbol as in the diagram. arsenic is a chemical element of the periodic table with chemical symbol as and. What Is The Valence Shell Configuration Of Arsenic.
From www.schoolmykids.com
Arsenic (As) Element Information, Facts, Properties, Uses Periodic What Is The Valence Shell Configuration Of Arsenic Therefore, the valence electrons of arsenic are five. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. arsenic has 5 valence electrons. The electron configuration shows that the last shell of arsenic has five electrons. The dots are placed on the four sides of the symbol, with one dot on each side and a fifth dot. What Is The Valence Shell Configuration Of Arsenic.
From periodictable.me
Arsenic Valence Electrons Arsenic Valency (As) with Dot Diagram What Is The Valence Shell Configuration Of Arsenic Ground state electron configuration for arsenic. Therefore, the valence electrons of arsenic are five. In the case of arsenic, there are 5 electrons in the outer or fourth shell. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. arsenic number of valence electrons. The dots are placed on the. What Is The Valence Shell Configuration Of Arsenic.
From commons.wikimedia.org
FileElectron shell 033 Arsenic.svg Wikimedia Commons What Is The Valence Shell Configuration Of Arsenic arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. The total number of electrons in a valence shell is called valence electrons. arsenic is a chemical element of the periodic table with chemical symbol as and. What Is The Valence Shell Configuration Of Arsenic.
From animalia-life.club
Electron Configuration Of Arsenic What Is The Valence Shell Configuration Of Arsenic the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the. the last shell after the electron configuration is called the valence shell. arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. Ground state electron configuration for arsenic. arsenic number of. What Is The Valence Shell Configuration Of Arsenic.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Arsenic (As) YouTube What Is The Valence Shell Configuration Of Arsenic The total number of electrons in a valence shell is called valence electrons. arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. These electrons are represented by dots surrounding the chemical symbol as in the diagram. In the case of arsenic, there are 5 electrons in the outer or. What Is The Valence Shell Configuration Of Arsenic.
From periodictable.me
Arsenic Electron Configuration (As) with Orbital Diagram What Is The Valence Shell Configuration Of Arsenic arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. These electrons are represented by dots. What Is The Valence Shell Configuration Of Arsenic.
From www.slideserve.com
PPT Chapter 8 Electron Configurations and Periodicity PowerPoint What Is The Valence Shell Configuration Of Arsenic Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. The dots are placed on the four sides of the symbol, with one dot on each side and a fifth dot placed between two sides. These electrons are represented by dots surrounding the chemical symbol as in the diagram. arsenic. What Is The Valence Shell Configuration Of Arsenic.
From www.examples.com
Arsenic (As) Definition, Preparation, Properties, Uses, Compounds What Is The Valence Shell Configuration Of Arsenic The total number of electrons in a valence shell is called valence electrons. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. Valence electrons are the number of electrons that are present at the. What Is The Valence Shell Configuration Of Arsenic.
From animalia-life.club
Electron Configuration Of Arsenic What Is The Valence Shell Configuration Of Arsenic arsenic, located in group 15 of the periodic table, has five valence electrons. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. In the case of arsenic, there are 5 electrons in the outer or fourth shell. The dots. What Is The Valence Shell Configuration Of Arsenic.
From valenceelectrons.com
Electron Configuration for Arsenic (As, As3 ion) What Is The Valence Shell Configuration Of Arsenic These electrons are represented by dots surrounding the chemical symbol as in the diagram. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. Therefore, the valence electrons of arsenic are five. arsenic has 5 valence electrons. the number of electrons in each element’s electron shells, particularly the outermost. What Is The Valence Shell Configuration Of Arsenic.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry What Is The Valence Shell Configuration Of Arsenic arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. In the case of arsenic, there are 5 electrons in the outer or fourth shell. These electrons are represented by dots surrounding the chemical symbol as in the diagram. arsenic number of valence electrons. the. What Is The Valence Shell Configuration Of Arsenic.
From www.pinterest.com
Arsenic, atomic structure Stock Image C018/3714 Science Photo What Is The Valence Shell Configuration Of Arsenic In the case of arsenic, there are 5 electrons in the outer or fourth shell. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the. arsenic number of valence electrons. The total number of electrons in a valence shell is called valence electrons. Ground state electron configuration for arsenic. arsenic is. What Is The Valence Shell Configuration Of Arsenic.
From periodictableguide.com
Arsenic (As) Periodic Table (Element Information & More) What Is The Valence Shell Configuration Of Arsenic the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. The dots are placed. What Is The Valence Shell Configuration Of Arsenic.
From www.schoolmykids.com
Arsenic (As) Element Information, Facts, Properties, Uses Periodic What Is The Valence Shell Configuration Of Arsenic arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. arsenic number of valence electrons. The electron configuration shows that the last shell of arsenic has five electrons. The total number of electrons in a valence shell is called valence electrons. arsenic has 5 valence. What Is The Valence Shell Configuration Of Arsenic.
From www.youtube.com
Write a noble gas or shorthand electron configuration arsenic YouTube What Is The Valence Shell Configuration Of Arsenic arsenic is a chemical element of the periodic table with chemical symbol as and atomic number 33 with an atomic weight of. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the. The. What Is The Valence Shell Configuration Of Arsenic.
From animalia-life.club
Electron Configuration Of Arsenic What Is The Valence Shell Configuration Of Arsenic The total number of electrons in a valence shell is called valence electrons. arsenic has five valence electrons in its outermost shell, and it can either gain three electrons to complete its. The electron configuration shows that the last shell of arsenic has five electrons. arsenic number of valence electrons. the number of electrons in each element’s. What Is The Valence Shell Configuration Of Arsenic.