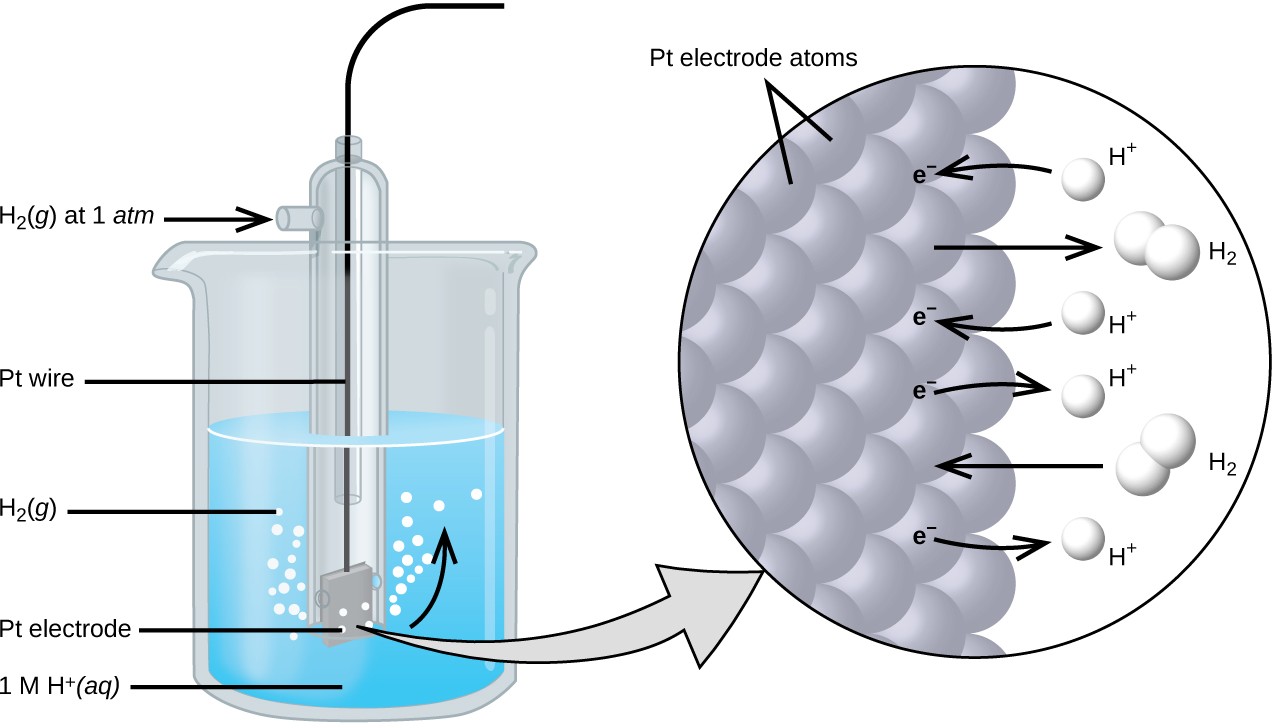
Why Hydrogen Electrode Is Used As Reference Electrode . this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. (i) reference electrodes of the first kind and (ii) reference electrodes of the second. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. standard hydrogen electrode. there are two types of reference electrodes: The structure of the standard hydrogen. Electrode potential is a measure of reducing power of any element. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common.
from psu.pb.unizin.org
in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. standard hydrogen electrode. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. there are two types of reference electrodes: although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. Electrode potential is a measure of reducing power of any element. The structure of the standard hydrogen. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. (i) reference electrodes of the first kind and (ii) reference electrodes of the second.
Electrode and Cell Potentials (17.3) Chemistry 110
Why Hydrogen Electrode Is Used As Reference Electrode this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. standard hydrogen electrode. although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. there are two types of reference electrodes: The structure of the standard hydrogen. (i) reference electrodes of the first kind and (ii) reference electrodes of the second. Electrode potential is a measure of reducing power of any element. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Why Hydrogen Electrode Is Used As Reference Electrode there are two types of reference electrodes: Electrode potential is a measure of reducing power of any element. standard hydrogen electrode. The structure of the standard hydrogen. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. this explains why. Why Hydrogen Electrode Is Used As Reference Electrode.
From mungfali.com
Reference Electrode Diagram Why Hydrogen Electrode Is Used As Reference Electrode (i) reference electrodes of the first kind and (ii) reference electrodes of the second. there are two types of reference electrodes: standard hydrogen electrode. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. The structure of the standard hydrogen. Electrode. Why Hydrogen Electrode Is Used As Reference Electrode.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use Why Hydrogen Electrode Is Used As Reference Electrode in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. The structure of the standard hydrogen. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. Electrode potential is a measure of reducing power of any element. values of e. Why Hydrogen Electrode Is Used As Reference Electrode.
From mavink.com
Hydrogen Electrode Diagram Why Hydrogen Electrode Is Used As Reference Electrode values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. there are two types of reference electrodes: The structure of the standard. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Why Hydrogen Electrode Is Used As Reference Electrode there are two types of reference electrodes: values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. (i) reference electrodes of the. Why Hydrogen Electrode Is Used As Reference Electrode.
From question.pandai.org
Standard Electrode Potential Why Hydrogen Electrode Is Used As Reference Electrode Electrode potential is a measure of reducing power of any element. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. The structure of. Why Hydrogen Electrode Is Used As Reference Electrode.
From gaskatel.de
Reference electrode Gaskatel Why Hydrogen Electrode Is Used As Reference Electrode although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. (i) reference electrodes of the first kind and (ii) reference electrodes of the second. The structure of the standard hydrogen.. Why Hydrogen Electrode Is Used As Reference Electrode.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Why Hydrogen Electrode Is Used As Reference Electrode The structure of the standard hydrogen. standard hydrogen electrode. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. although the. Why Hydrogen Electrode Is Used As Reference Electrode.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Why Hydrogen Electrode Is Used As Reference Electrode (i) reference electrodes of the first kind and (ii) reference electrodes of the second. Electrode potential is a measure of reducing power of any element. standard hydrogen electrode. The structure of the standard hydrogen. although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. there are two types. Why Hydrogen Electrode Is Used As Reference Electrode.
From byjus.com
What is standard hydrogen electrode? Why Hydrogen Electrode Is Used As Reference Electrode Electrode potential is a measure of reducing power of any element. there are two types of reference electrodes: The structure of the standard hydrogen. (i) reference electrodes of the first kind and (ii) reference electrodes of the second. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard. Why Hydrogen Electrode Is Used As Reference Electrode.
From psu.pb.unizin.org
Electrode and Cell Potentials (17.3) Chemistry 110 Why Hydrogen Electrode Is Used As Reference Electrode Electrode potential is a measure of reducing power of any element. The structure of the standard hydrogen. there are two types of reference electrodes: standard hydrogen electrode. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Why Hydrogen Electrode Is Used As Reference Electrode although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. standard hydrogen electrode. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. values of e 0 are most often reported as the potential measured in an electrochemical cell. Why Hydrogen Electrode Is Used As Reference Electrode.
From askfilo.com
Standard hydrogen electrode (SHE) It is used as reference electrode who.. Why Hydrogen Electrode Is Used As Reference Electrode Electrode potential is a measure of reducing power of any element. The structure of the standard hydrogen. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. standard hydrogen electrode.. Why Hydrogen Electrode Is Used As Reference Electrode.
From stock.adobe.com
Vetor de A Standard Hydrogen Electrode or SHE is an electrode that Why Hydrogen Electrode Is Used As Reference Electrode there are two types of reference electrodes: standard hydrogen electrode. although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. The structure of the standard hydrogen. (i) reference electrodes of the first kind and (ii) reference electrodes of the second. in electrochemistry, the standard hydrogen electrode (abbreviated. Why Hydrogen Electrode Is Used As Reference Electrode.
From gamesmartz.com
Standard Hydrogen Electrode Definition & Image GameSmartz Why Hydrogen Electrode Is Used As Reference Electrode in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. standard hydrogen electrode. The structure of the standard hydrogen. there are two types of reference electrodes: values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.askiitians.com
Electrode Potential Study Material for IIT JEE askIITians Why Hydrogen Electrode Is Used As Reference Electrode in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. standard hydrogen electrode. The structure of the standard hydrogen. Electrode potential is a measure of reducing power of any element. there are two types of reference electrodes: this explains why the reduction and oxidation reactions associated with a. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Why Hydrogen Electrode Is Used As Reference Electrode although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. The structure of the standard hydrogen. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. (i) reference electrodes of the first kind and (ii) reference electrodes of the second. . Why Hydrogen Electrode Is Used As Reference Electrode.
From www.numerade.com
SOLVED (This is for a chemical engineering class) Standard hydrogen Why Hydrogen Electrode Is Used As Reference Electrode Electrode potential is a measure of reducing power of any element. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. standard hydrogen electrode. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. although the standard hydrogen electrode. Why Hydrogen Electrode Is Used As Reference Electrode.
From monomole.com
Standard hydrogen electrode Mono Mole Why Hydrogen Electrode Is Used As Reference Electrode this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. standard hydrogen electrode. Electrode potential is a measure of reducing power of any element. although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. The structure of the standard. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.vedantu.com
Define the reference electrode. Why Hydrogen Electrode Is Used As Reference Electrode values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. there are two types of reference electrodes: (i) reference electrodes of the first. Why Hydrogen Electrode Is Used As Reference Electrode.
From mungfali.com
Types Of Electrodes Why Hydrogen Electrode Is Used As Reference Electrode (i) reference electrodes of the first kind and (ii) reference electrodes of the second. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. there are two types of reference electrodes: although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is. Why Hydrogen Electrode Is Used As Reference Electrode.
From gaskatel.de
HydroFlex® Hydrogen Reference Electrode Gaskatel Why Hydrogen Electrode Is Used As Reference Electrode this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.slideserve.com
PPT Introduction to electrochemical techniques PowerPoint Why Hydrogen Electrode Is Used As Reference Electrode The structure of the standard hydrogen. standard hydrogen electrode. Electrode potential is a measure of reducing power of any element. (i) reference electrodes of the first kind and (ii) reference electrodes of the second. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. there are two types. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 9 Nernst Equation PowerPoint Why Hydrogen Electrode Is Used As Reference Electrode although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. (i) reference electrodes of the first kind and (ii) reference electrodes of the second. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as. Why Hydrogen Electrode Is Used As Reference Electrode.
From avopix.com
Standard hydrogen electrode diagram. Scientific Royalty Free Stock Why Hydrogen Electrode Is Used As Reference Electrode there are two types of reference electrodes: in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. although the standard hydrogen electrode is the reference electrode used to define. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.youtube.com
Why Hydrogen electrode acts as both cathode and anode SHE as a Why Hydrogen Electrode Is Used As Reference Electrode in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. standard hydrogen electrode. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. (i) reference electrodes of the first kind and (ii) reference. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.youtube.com
Standard Hydrogen Electrode YouTube Why Hydrogen Electrode Is Used As Reference Electrode there are two types of reference electrodes: values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. standard hydrogen electrode. Electrode potential is a measure of reducing power of any element. (i) reference electrodes of the first kind and (ii) reference. Why Hydrogen Electrode Is Used As Reference Electrode.
From chemwiki.ucdavis.edu
11B Potentiometric Methods Chemwiki Why Hydrogen Electrode Is Used As Reference Electrode there are two types of reference electrodes: although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. (i) reference electrodes of the first kind and (ii) reference electrodes of the. Why Hydrogen Electrode Is Used As Reference Electrode.
From saylordotorg.github.io
Electrochemistry Why Hydrogen Electrode Is Used As Reference Electrode although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. The structure of the standard hydrogen. (i) reference electrodes of the first kind and (ii) reference electrodes of the second.. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Why Hydrogen Electrode Is Used As Reference Electrode in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. there are two types of reference electrodes: The structure of the standard hydrogen. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a.. Why Hydrogen Electrode Is Used As Reference Electrode.
From gaskatel.de
Usage of the hydrogen electrodes HydroFlex and MiniHydroFlex Gaskatel Why Hydrogen Electrode Is Used As Reference Electrode this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. values of e 0 are most often reported as the potential measured in an electrochemical cell for which the. Why Hydrogen Electrode Is Used As Reference Electrode.
From saylordotorg.github.io
Standard Potentials Why Hydrogen Electrode Is Used As Reference Electrode in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. although the standard hydrogen electrode is the reference electrode used to define electrode potentials, it use is not common. The structure of the standard hydrogen. Electrode potential is a measure of reducing power of any element. values of e. Why Hydrogen Electrode Is Used As Reference Electrode.
From www.lambdasystem.pl
Hydrogen reference electrode Electrodes ELECTROCHEMISTRY Why Hydrogen Electrode Is Used As Reference Electrode Electrode potential is a measure of reducing power of any element. (i) reference electrodes of the first kind and (ii) reference electrodes of the second. there are two types of reference electrodes: this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. although the standard hydrogen electrode is. Why Hydrogen Electrode Is Used As Reference Electrode.
From dxodcnqen.blob.core.windows.net
Standard Hydrogen Electrode In A Galvanic Cell at Katharine Murphy blog Why Hydrogen Electrode Is Used As Reference Electrode The structure of the standard hydrogen. there are two types of reference electrodes: standard hydrogen electrode. in electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. (i) reference electrodes of the first kind and (ii) reference electrodes of the second. this explains why the reduction and oxidation reactions. Why Hydrogen Electrode Is Used As Reference Electrode.
From mungfali.com
Ppt Chapter 17 Electrochemistry Powerpoint Presentation, Free A12 Why Hydrogen Electrode Is Used As Reference Electrode values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. this explains why the reduction and oxidation reactions associated with a given redox couple selected for a reference electrode. there are two types of reference electrodes: although the standard hydrogen. Why Hydrogen Electrode Is Used As Reference Electrode.