Amino Acids With Side Chain Pka . Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. At neutral ph the amino group is protonated, and the carboxyl group is. Basic amino acids with a basic side chain are hydrophilic. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present.
from www.chegg.com
Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Basic amino acids with a basic side chain are hydrophilic. At neutral ph the amino group is protonated, and the carboxyl group is. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present.
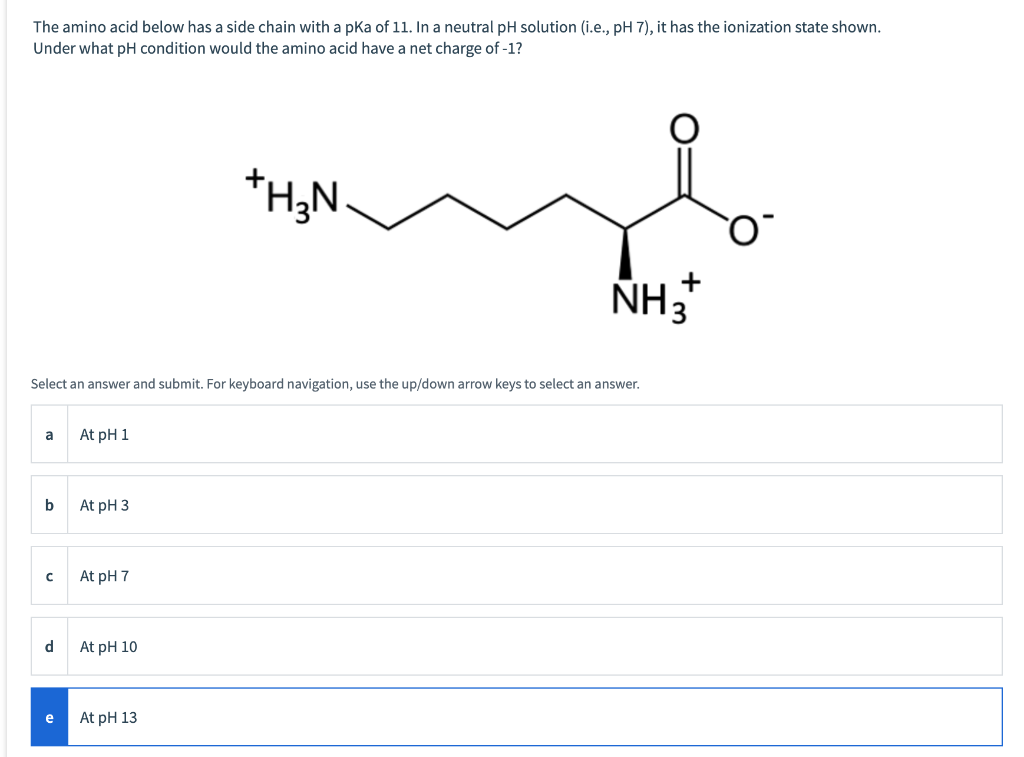
Solved The side chain for the amino acid shown has a pKa of
Amino Acids With Side Chain Pka Basic amino acids with a basic side chain are hydrophilic. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. At neutral ph the amino group is protonated, and the carboxyl group is. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. Basic amino acids with a basic side chain are hydrophilic. For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present.
From www.researchgate.net
The pKa of the three alkaline amino acids (Arg, Lys and His) and the... Download Table Amino Acids With Side Chain Pka These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. For amino acids with ionizable side chains, determining the pi involves. Amino Acids With Side Chain Pka.
From bradleyabbott.z13.web.core.windows.net
Amino Acid Side Chain Chart Amino Acids With Side Chain Pka Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. Basic amino acids with a basic side chain are hydrophilic. Pka values for amino acid side chains are very dependent upon the. Amino Acids With Side Chain Pka.
From www.chegg.com
Solved 3) Which amino acid has a sidechain with a pka value Amino Acids With Side Chain Pka For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. At neutral ph the amino group is protonated, and the carboxyl group is. These amino acids have basic primary or secondary. Amino Acids With Side Chain Pka.
From truevaluedentalinstitute.in
Classification of Side Chains of Amino Acids True value dental Institute Amino Acids With Side Chain Pka For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Basic amino acids with a basic side chain are hydrophilic. At neutral ph the amino group is protonated, and the carboxyl group is. These amino acids have basic primary or secondary amine groups in their side chain that ionize to. Amino Acids With Side Chain Pka.
From www.chegg.com
Solved The side chain for the amino acid shown has a pKa of Amino Acids With Side Chain Pka At neutral ph the amino group is protonated, and the carboxyl group is. For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Basic amino acids with a basic side chain are hydrophilic. Pka values for amino acid side chains are very dependent upon the chemical environment in which they. Amino Acids With Side Chain Pka.
From www.reagent.co.uk
What Are Amino Acids? The Science Blog Amino Acids With Side Chain Pka At neutral ph the amino group is protonated, and the carboxyl group is. For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Basic amino acids with a basic side chain. Amino Acids With Side Chain Pka.
From www.researchgate.net
Amino acids with ionizable side chains in enzymes a . Download Scientific Diagram Amino Acids With Side Chain Pka At neutral ph the amino group is protonated, and the carboxyl group is. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. If the amino acid has a side chain. Amino Acids With Side Chain Pka.
From www.vrogue.co
How To Use A Pka Table vrogue.co Amino Acids With Side Chain Pka Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. Pka values for amino acid side chains are very dependent upon. Amino Acids With Side Chain Pka.
From amit1b.wordpress.com
Amino Acids Chemistry, Biochemistry & Nutrition Amit Kessel Ph.D Amino Acids With Side Chain Pka At neutral ph the amino group is protonated, and the carboxyl group is. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. These amino acids have basic primary or secondary amine groups in their side chain that ionize to. Amino Acids With Side Chain Pka.
From jackwestin.com
Complete MCAT Amino Acids Proteins Guide MCAT Content Amino Acids With Side Chain Pka Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. Basic amino acids with a basic side chain are hydrophilic. For. Amino Acids With Side Chain Pka.
From www.doubtnut.com
If an amino acid has ionizable side chain, its pl is the average of the pka values of the Amino Acids With Side Chain Pka At neutral ph the amino group is protonated, and the carboxyl group is. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Basic amino acids with a basic side chain are. Amino Acids With Side Chain Pka.
From www.researchgate.net
Amino Acid pKa and corresponding protonation states at low, medium, and... Download Scientific Amino Acids With Side Chain Pka Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Basic amino acids with a basic side chain are hydrophilic. At neutral ph the amino group is protonated, and the carboxyl group. Amino Acids With Side Chain Pka.
From www.quora.com
What is the side chain of an amino acid? Quora Amino Acids With Side Chain Pka At neutral ph the amino group is protonated, and the carboxyl group is. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Basic amino acids with a basic side chain are hydrophilic. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are. Amino Acids With Side Chain Pka.
From www.reddit.com
Amino acids pKa question r/Mcat Amino Acids With Side Chain Pka For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Basic amino acids with a basic side chain are hydrophilic. At neutral ph the amino group is protonated, and the carboxyl group is. Pka values for amino acid side chains are very dependent upon the chemical environment in which they. Amino Acids With Side Chain Pka.
From www.chegg.com
Solved Problem 17.8 Part A The PKa Values Of Amino Acids Amino Acids With Side Chain Pka Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. For amino acids. Amino Acids With Side Chain Pka.
From world-wallpaper.blogspot.com
. pka of amino acids Amino Acids With Side Chain Pka Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. Basic amino acids with a basic side chain are hydrophilic. For amino acids with ionizable side chains, determining the pi involves considering. Amino Acids With Side Chain Pka.
From www.organicchemistrytutor.com
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic Chemistry Tutor Amino Acids With Side Chain Pka Basic amino acids with a basic side chain are hydrophilic. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. For. Amino Acids With Side Chain Pka.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Organic Chemistry Amino Acids With Side Chain Pka Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. These amino acids have basic primary or secondary amine groups in. Amino Acids With Side Chain Pka.
From wou.edu
Chapter 2 Protein Structure Chemistry Amino Acids With Side Chain Pka For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. At neutral ph the amino group is protonated, and the carboxyl group is. Basic amino acids with a basic side chain are hydrophilic. These amino acids have basic primary or secondary amine groups in their side chain that ionize to. Amino Acids With Side Chain Pka.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation ID226444 Amino Acids With Side Chain Pka Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Basic amino acids with a basic side chain are hydrophilic. For amino acids with ionizable side chains, determining the pi involves considering. Amino Acids With Side Chain Pka.
From www.pinterest.jp
20 Different Amino Acids structure to it Twenty different types of side chains (20 amino Amino Acids With Side Chain Pka Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. At neutral ph the amino group is protonated, and the carboxyl group is. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average. Amino Acids With Side Chain Pka.
From www.researchgate.net
Amino Acid pKa and corresponding protonation states at low, medium, and... Download Scientific Amino Acids With Side Chain Pka These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. At neutral ph the amino group is protonated, and the carboxyl. Amino Acids With Side Chain Pka.
From www.chegg.com
Solved The side chain for the amino acid shown has a pKa of Amino Acids With Side Chain Pka For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. At neutral ph the amino group is protonated, and the carboxyl group is. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Basic amino acids with a basic side chain. Amino Acids With Side Chain Pka.
From www.chegg.com
Solved 4. The pka of an amino acid's side chain is Amino Acids With Side Chain Pka If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Basic amino acids with a basic side chain are hydrophilic. For. Amino Acids With Side Chain Pka.
From www.slideshare.net
Lecture 2 3 protein chemistry Amino Acids With Side Chain Pka For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Pka values. Amino Acids With Side Chain Pka.
From www.researchgate.net
Structure of amino acid side chains group ([44]). Download Scientific Diagram Amino Acids With Side Chain Pka If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Pka values for amino acid side chains are very dependent upon. Amino Acids With Side Chain Pka.
From leah4sci.com
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial Amino Acids With Side Chain Pka At neutral ph the amino group is protonated, and the carboxyl group is. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. These amino acids have basic primary or secondary amine. Amino Acids With Side Chain Pka.
From www.masterorganicchemistry.com
AcidBase Reactions Introducing Ka and pKa Master Organic Chemistry Amino Acids With Side Chain Pka For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. At neutral ph the amino group is protonated, and the carboxyl group is. If the amino acid has a side chain. Amino Acids With Side Chain Pka.
From www.chegg.com
Solved Consider The Side Chain Of The Amino Acid Histidin... Amino Acids With Side Chain Pka If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Pka values for amino acid side chains are very dependent. Amino Acids With Side Chain Pka.
From solvedlib.com
The side chain pKa of the amino acids shown in the … SolvedLib Amino Acids With Side Chain Pka Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. At neutral ph the amino group is protonated, and the carboxyl group is. Pka values for amino acid side chains are very. Amino Acids With Side Chain Pka.
From www.numerade.com
SOLVED Amino Acid pKa of aCOOH pKa of aNH2 pKa of Side Chain Isoelectric Point (pI) Alanine 2 Amino Acids With Side Chain Pka For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. If the. Amino Acids With Side Chain Pka.
From www.sampletemplates.com
16+ Amino Acid Chart Templates Sample Templates Amino Acids With Side Chain Pka At neutral ph the amino group is protonated, and the carboxyl group is. If the amino acid has a side chain that also has a pka (pka 3 in the table above), the isoelectric point is determined by taking the average of the. For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino. Amino Acids With Side Chain Pka.
From www.numerade.com
table 232 the pka values of amino acids pka amino acid m cooh pka a nh pka side chain alanine Amino Acids With Side Chain Pka These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. For amino acids. Amino Acids With Side Chain Pka.
From peptideweb.com
Amino acid properties Amino Acids With Side Chain Pka For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Pka values for amino acid side chains are very dependent upon the chemical environment in which they are present. At neutral ph the amino group is protonated, and the carboxyl group is. Basic amino acids with a basic side chain. Amino Acids With Side Chain Pka.
From www.pinterest.com
Image result for amino acid side chain pka Biology Review, Mcat Study, Functional Group, Acide Amino Acids With Side Chain Pka At neutral ph the amino group is protonated, and the carboxyl group is. For amino acids with ionizable side chains, determining the pi involves considering the ionization of the amino and carboxyl groups,. Basic amino acids with a basic side chain are hydrophilic. Pka values for amino acid side chains are very dependent upon the chemical environment in which they. Amino Acids With Side Chain Pka.