Gas Laws Of Syringes . Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. The body as well as the mechanism of action of drugs (e.g., inhaled. Explore the principles of gas laws and their clinical applications in medicine. Understand how boyle's law, charles's law, gay.
from www.shutterstock.com
While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. Understand how boyle's law, charles's law, gay. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. Explore the principles of gas laws and their clinical applications in medicine. The body as well as the mechanism of action of drugs (e.g., inhaled. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k).
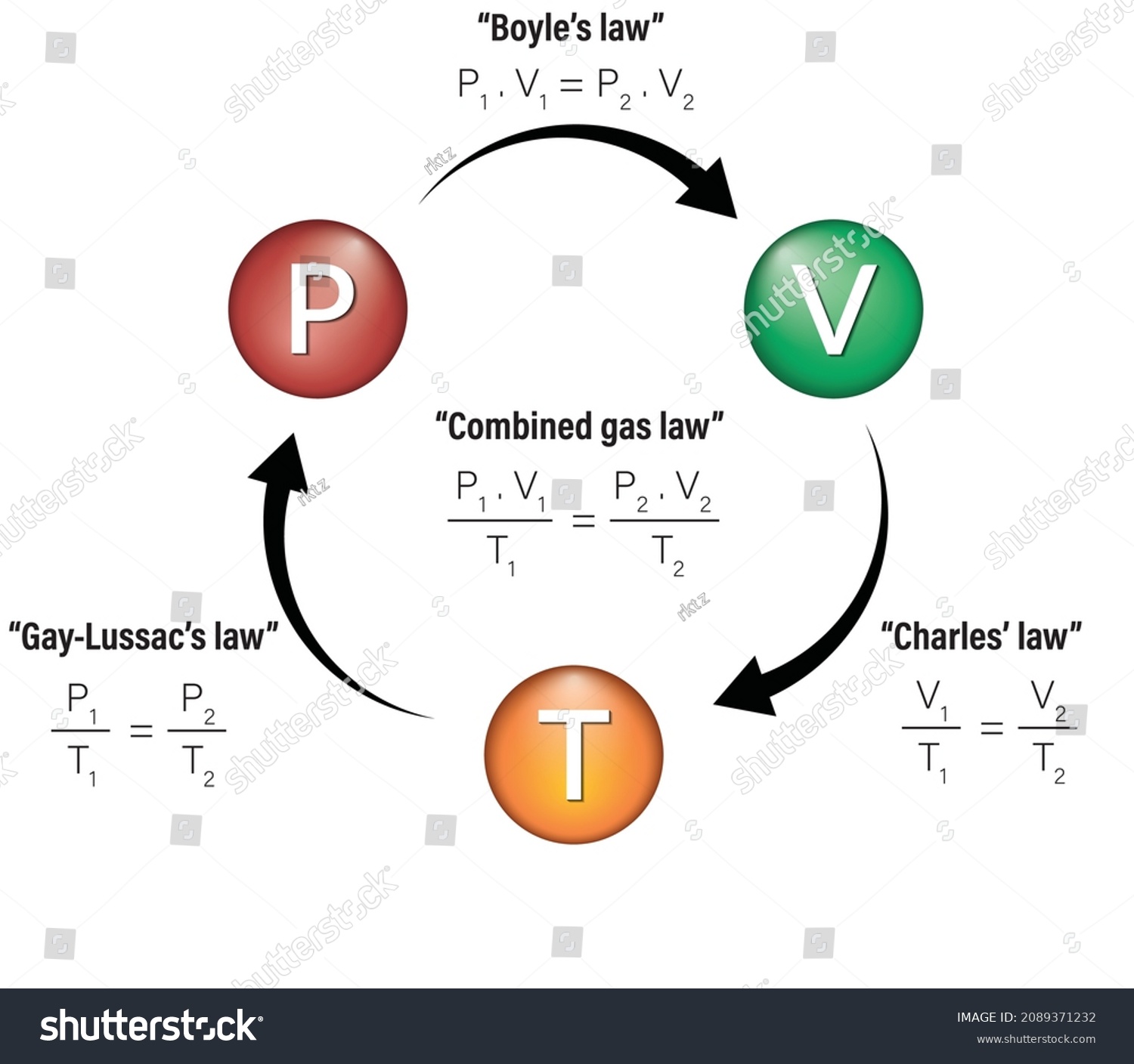
Gas Laws Infographic Diagram Showing Combined vetor stock (livre de
Gas Laws Of Syringes The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. Understand how boyle's law, charles's law, gay. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. The body as well as the mechanism of action of drugs (e.g., inhaled. Explore the principles of gas laws and their clinical applications in medicine. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed.
From scienceducatio.weebly.com
Gas Laws Environmental Chemistry Gas Laws Of Syringes Explore the principles of gas laws and their clinical applications in medicine. While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Before setting up the apparatus as shown in the diagram. Gas Laws Of Syringes.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube Gas Laws Of Syringes Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. Understand how boyle's law, charles's law, gay. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. The pressure of a fixed. Gas Laws Of Syringes.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Gas Laws Of Syringes Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. Understand how boyle's law, charles's law, gay. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. While many of these laws apply to ‘ideal’ gases in closed systems at standard. Gas Laws Of Syringes.
From www.indiamart.com
Gas Chromatography Syringes at Rs 1100 Kalbadevi Mumbai ID Gas Laws Of Syringes The body as well as the mechanism of action of drugs (e.g., inhaled. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. Understand how boyle's law, charles's law, gay. While many of these laws apply to ‘ideal’ gases in closed. Gas Laws Of Syringes.
From www.numerade.com
SOLVED Hi, what are the reallife applications and examples of the gas Gas Laws Of Syringes Understand how boyle's law, charles's law, gay. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Explore the principles of gas laws and their clinical applications in medicine. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be. Gas Laws Of Syringes.
From syringepumppro.com
Parts Of A Syringe SyringePumpPro Gas Laws Of Syringes Understand how boyle's law, charles's law, gay. The body as well as the mechanism of action of drugs (e.g., inhaled. Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. Explore the principles of gas laws and their clinical applications in medicine. Before setting up the apparatus as shown in. Gas Laws Of Syringes.
From www.homeworkminutes.com
What are the Different Gas Laws in Chemistry Gas Laws Of Syringes The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Explore the principles of gas laws and their clinical applications in medicine. While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. The body as well as the mechanism of action of. Gas Laws Of Syringes.
From www.numerade.com
SOLVED Potentially Useful Information Dalton'Law of Partial Pressure Gas Laws Of Syringes Understand how boyle's law, charles's law, gay. The body as well as the mechanism of action of drugs (e.g., inhaled. Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. The. Gas Laws Of Syringes.
From alchetron.com
Gas syringe Alchetron, The Free Social Encyclopedia Gas Laws Of Syringes The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. Describes a number of experiments and demonstrations that rely on. Gas Laws Of Syringes.
From hxeyewyyy.blob.core.windows.net
Syringes Gas Laws at Lynn Lewis blog Gas Laws Of Syringes The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. Explore the principles of gas laws and their clinical applications in medicine. Describes a number of experiments and demonstrations that. Gas Laws Of Syringes.
From www.studocu.com
Gas law comparison table GAS LAW WORKING FORMULA CONSTANT Gas Laws Of Syringes While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. Explore the principles of gas laws and their clinical applications in medicine. Understand how boyle's law, charles's law, gay. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. The pressure of a fixed. Gas Laws Of Syringes.
From www.studypool.com
SOLUTION Gas laws key worksheet with answer Studypool Gas Laws Of Syringes Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. The ideal gas law syringe allows simultaneous measurements of temperature and. Gas Laws Of Syringes.
From www.studocu.com
Gas Laws Packet without Answers AP* Chemistry GASES The gaseous state Gas Laws Of Syringes Understand how boyle's law, charles's law, gay. The body as well as the mechanism of action of drugs (e.g., inhaled. Explore the principles of gas laws and their clinical applications in medicine. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Describes a number of experiments and demonstrations. Gas Laws Of Syringes.
From www.britannica.com
Boyle’s law Definition, Equation, & Facts Britannica Gas Laws Of Syringes Explore the principles of gas laws and their clinical applications in medicine. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Understand how boyle's law, charles's law, gay. The body as well as the mechanism of action of drugs (e.g., inhaled. While many of these laws apply to. Gas Laws Of Syringes.
From www.worksheetsplanet.com
The Gas Laws Gas Laws Of Syringes Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. While many of these laws apply to ‘ideal’ gases in closed. Gas Laws Of Syringes.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Gas Laws Of Syringes The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. Understand how boyle's law, charles's law, gay. Explore the principles of gas laws and their clinical applications in medicine. The body as well as the mechanism of action of drugs (e.g., inhaled. The pressure of a fixed mass of gas at. Gas Laws Of Syringes.
From www.shutterstock.com
Gas Laws Infographic Diagram Showing Combined vetor stock (livre de Gas Laws Of Syringes Understand how boyle's law, charles's law, gay. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. Before setting up the apparatus as shown in the diagram the inside diameter, d of. Gas Laws Of Syringes.
From www.researchgate.net
The syringe and the method of taking the required volume from the gas Gas Laws Of Syringes The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. Before setting up the. Gas Laws Of Syringes.
From hxeyewyyy.blob.core.windows.net
Syringes Gas Laws at Lynn Lewis blog Gas Laws Of Syringes Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. Understand how boyle's law, charles's law, gay. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. While many of these. Gas Laws Of Syringes.
From www.hamiltoncompany.com
Gas Tight Syringes Laboratory Hamilton Gastight Syringes Gas Laws Of Syringes Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. Understand how boyle's law, charles's law, gay. While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. The ideal gas law syringe allows simultaneous. Gas Laws Of Syringes.
From gootutorials.blogspot.com
How To Use A Gas Syringe To Measure Gas Gas Laws Of Syringes The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Explore the principles of gas laws and their clinical applications in medicine. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing. Gas Laws Of Syringes.
From www.youtube.com
BOYLE'S LAW OF IDEAL GASSES (Syringes x balloons) YouTube Gas Laws Of Syringes While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Before setting up. Gas Laws Of Syringes.
From www.youtube.com
112 Syringe Pressures (Boyle's Law) YouTube Gas Laws Of Syringes Explore the principles of gas laws and their clinical applications in medicine. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Understand how boyle's law, charles's law, gay. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be. Gas Laws Of Syringes.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii Gas Laws Of Syringes Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. The body as well as the mechanism of action of drugs (e.g., inhaled. Understand how boyle's law, charles's law, gay. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. Explore. Gas Laws Of Syringes.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal Gas Laws Of Syringes While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. The body as well as the mechanism of action of drugs (e.g., inhaled. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. Understand how boyle's law, charles's law, gay. The pressure of a. Gas Laws Of Syringes.
From www.chegg.com
Solved You will use syringes in the experiment to change the Gas Laws Of Syringes Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. Explore the principles of gas laws and their clinical applications in medicine.. Gas Laws Of Syringes.
From tomi.digital
TOMi.digital Gas Laws Part II Gas Laws Of Syringes While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. The pressure of a. Gas Laws Of Syringes.
From pngmoua.blogspot.com
Measurement Reading Syringes Worksheet / Open Lesson 3 The Gas Laws Gas Laws Of Syringes Explore the principles of gas laws and their clinical applications in medicine. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger.. Gas Laws Of Syringes.
From www.philipharris.co.uk
B8A36171 Gas Syringe 100cm³ Philip Harris Gas Laws Of Syringes The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. The body as. Gas Laws Of Syringes.
From www.youtube.com
Gas Syringe YouTube Gas Laws Of Syringes Understand how boyle's law, charles's law, gay. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. Describes a number of experiments and demonstrations that rely on the use of. Gas Laws Of Syringes.
From mmerevise.co.uk
The Ideal Gas Equation MME Gas Laws Of Syringes The body as well as the mechanism of action of drugs (e.g., inhaled. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Explore the principles of gas laws and their clinical applications in medicine. While many of these laws apply to ‘ideal’ gases in closed systems at standard. Gas Laws Of Syringes.
From www.studocu.com
Assignment of Combined Gas Laws Combined Gas Laws A gas occupies a Gas Laws Of Syringes The body as well as the mechanism of action of drugs (e.g., inhaled. While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. The. Gas Laws Of Syringes.
From facts.net
19 Mindblowing Facts About Gas Laws Gas Laws Of Syringes The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's law, charles' law,. Explore the. Gas Laws Of Syringes.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal Gas Laws Of Syringes Understand how boyle's law, charles's law, gay. The ideal gas law syringe allows simultaneous measurements of temperature and pressure of a gas as it is compressed. While many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure. Describes a number of experiments and demonstrations that rely on the use of hypodermic syringes, including boyle's. Gas Laws Of Syringes.
From www.vectorstock.com
Ideal gas law boyles law pressure volume Vector Image Gas Laws Of Syringes Understand how boyle's law, charles's law, gay. The pressure of a fixed mass of gas at constant volume is directly proportional to its absolute temperature (p/t = k). Before setting up the apparatus as shown in the diagram the inside diameter, d of the syringe needs to be measured using a vernier calliper after removing the plunger. The ideal gas. Gas Laws Of Syringes.