Tablet Dissolution Definition . Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines. Dissolution is the process in which a substance forms a solution. In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. The administration of drugs via oral dosage forms is one of the most common and effective means of delivering. The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates when the. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. As a quality control test, the dissolution test is. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for.
from www.mdpi.com
Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. As a quality control test, the dissolution test is. Dissolution is the process in which a substance forms a solution. The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates when the. In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. The administration of drugs via oral dosage forms is one of the most common and effective means of delivering.
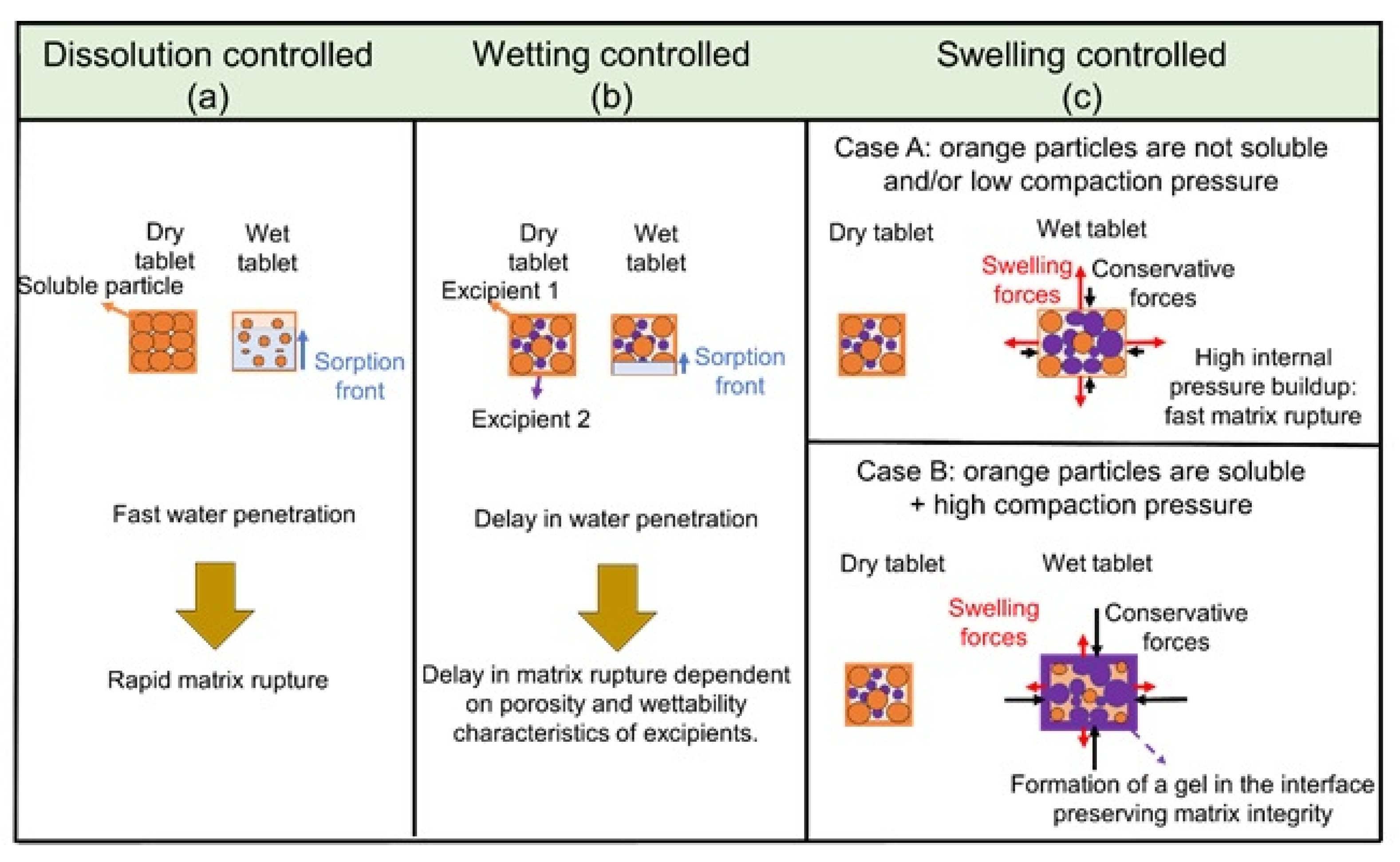
Powders Free FullText The Significance of Tablet Internal
Tablet Dissolution Definition Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines. The administration of drugs via oral dosage forms is one of the most common and effective means of delivering. Dissolution is the process in which a substance forms a solution. The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates when the. In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. As a quality control test, the dissolution test is.
From www.labkafe.com
Tablet Dissolution Apparatus Labkafe Tablet Dissolution Definition Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. As. Tablet Dissolution Definition.
From www.sciencephoto.com
Indigestion tablet dissolving in water Stock Image M635/0038 Tablet Dissolution Definition As a quality control test, the dissolution test is. The administration of drugs via oral dosage forms is one of the most common and effective means of delivering. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. The dissolution test in a usp drug product monograph helps evaluate the performance of. Tablet Dissolution Definition.
From uvison.com
Tablet Dissolution System 14000 (Basic) SMART Tablet Dissolution Definition Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes,. Tablet Dissolution Definition.
From www.researchgate.net
SEM images of tablet dissolution at high magnification. All images Tablet Dissolution Definition The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates when the. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines.. Tablet Dissolution Definition.
From www.slideserve.com
PPT Comparative dissolution testing and applications PowerPoint Tablet Dissolution Definition In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different. Tablet Dissolution Definition.
From www.thoughtco.com
Dissolve Definition in Chemistry Tablet Dissolution Definition Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. As a quality control test, the dissolution test is. Dissolution testing measures the extent and rate of solution formation. Tablet Dissolution Definition.
From www.thoughtco.com
Dissociation Reaction Definition and Examples Tablet Dissolution Definition Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. As a quality control. Tablet Dissolution Definition.
From www.mdpi.com
Powders Free FullText The Significance of Tablet Internal Tablet Dissolution Definition Dissolution is the process in which a substance forms a solution. Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. Dissolution testing is an important physiochemical test for the development of. Tablet Dissolution Definition.
From www.researchgate.net
The dissolution profiles in different medium of the two tablet Tablet Dissolution Definition In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates when the. The administration of drugs via oral dosage forms is one of the most common and. Tablet Dissolution Definition.
From www.chromatechscientific.com
Next Generation of Tablet dissolution apparatus Tablet Dissolution Definition As a quality control test, the dissolution test is. Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines. The administration of drugs via oral dosage forms is one of the most common and effective means of delivering. Dissolution testing provides critical information to support the realisation of drug release. Tablet Dissolution Definition.
From auto-labsystems.co.uk
Tablet Dissolution Testing Automatic Lab Systems Ltd Tablet Dissolution Definition In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. As a quality control test, the dissolution test is. Dissolution is the process in which a substance forms a solution. The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product. Tablet Dissolution Definition.
From www.slideserve.com
PPT The International Pharmacopoeia Quality standards for TB Tablet Dissolution Definition As a quality control test, the dissolution test is. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines. The dissolution test in a usp drug product monograph helps evaluate the performance. Tablet Dissolution Definition.
From etconanalytical.com
Tablet Dissolution Testing Instruments Etcon Analytical and Tablet Dissolution Definition Dissolution is the process in which a substance forms a solution. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. The administration of drugs. Tablet Dissolution Definition.
From www.slideserve.com
PPT Quality control of tablets PowerPoint Presentation, free download Tablet Dissolution Definition Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. The dissolution test in a usp drug product monograph helps evaluate the performance of a. Tablet Dissolution Definition.
From www.slideserve.com
PPT Statistical Design of Experiments PowerPoint Presentation, free Tablet Dissolution Definition The administration of drugs via oral dosage forms is one of the most common and effective means of delivering. Dissolution is the process in which a substance forms a solution. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. Dissolution limits for ir, dr and er solid oral dosage form for. Tablet Dissolution Definition.
From www.youtube.com
Evaluation of tablet, Dissolution test YouTube Tablet Dissolution Definition Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. The administration of drugs via oral dosage forms is one of the most common and effective means of delivering.. Tablet Dissolution Definition.
From www.pginstruments.com
Tablet Dissolution PG Instruments Limited Tablet Dissolution Definition The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates when the. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. The administration of drugs via oral dosage forms is one of the most common and effective means of delivering. Dissolution. Tablet Dissolution Definition.
From www.dreamstime.com
Tablet Dissolving In A Glass O Stock Photography Image 2678882 Tablet Dissolution Definition In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. The administration of drugs via oral dosage forms is one of the most common and effective means of delivering. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,.. Tablet Dissolution Definition.
From www.researchgate.net
Evolution of dissolution of tablets after manufacturing and after Tablet Dissolution Definition In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. Dissolution is the process in which a substance forms a. Tablet Dissolution Definition.
From www.slideserve.com
PPT Solid Dosage Forms (Tablets) PowerPoint Presentation, free Tablet Dissolution Definition As a quality control test, the dissolution test is. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. The dissolution test in a usp. Tablet Dissolution Definition.
From www.chromatechscientific.com
TABLET DISSOLUTION chromatechscientifi Tablet Dissolution Definition As a quality control test, the dissolution test is. The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates when the. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. Dissolution is. Tablet Dissolution Definition.
From www.researchgate.net
Tablet dissolution apparatus parameters Download Scientific Diagram Tablet Dissolution Definition In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. As a quality control test, the dissolution test is. Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines. The administration of drugs via oral dosage forms. Tablet Dissolution Definition.
From www.bruker.com
Dissolution of a tablets Bruker Tablet Dissolution Definition Dissolution is the process in which a substance forms a solution. As a quality control test, the dissolution test is. The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates when the. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of. Tablet Dissolution Definition.
From www.youtube.com
DISSOLUTION TEST FOR TABLET DOSAGE FORM TABLET EVALUTION PARAMETER Tablet Dissolution Definition In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. Dissolution is the process in which a substance forms a. Tablet Dissolution Definition.
From www.slidemake.com
Dissolution Of Tablets Presentation Tablet Dissolution Definition Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. As a quality control test, the dissolution test is. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. Dissolution is the process in which. Tablet Dissolution Definition.
From www.researchgate.net
Dissolution profiles for tablet prepared by direct compression Tablet Dissolution Definition Dissolution is the process in which a substance forms a solution. The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates when the. The administration of drugs via oral dosage forms is one of the most common and effective means of delivering. Dissolution testing provides critical information to support the. Tablet Dissolution Definition.
From www.researchgate.net
Changes to internal structures of the whole tablet during drug Tablet Dissolution Definition Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines. In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is. Tablet Dissolution Definition.
From fr.dreamstime.com
Dissoudre De Tablette Image stock Image 4535841 Tablet Dissolution Definition As a quality control test, the dissolution test is. The dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates when the. In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. Dissolution testing is an important physiochemical. Tablet Dissolution Definition.
From www.researchgate.net
Dissolution profile of control tablet, tablets containing sodium starch Tablet Dissolution Definition Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. As a quality control test, the dissolution test is. In vivo, the disintegration and dissolution. Tablet Dissolution Definition.
From www.pharmaexcipients.com
Evaluation of dissolution techniques for orally disintegrating mini Tablet Dissolution Definition In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. As a quality control test, the dissolution test is. Dissolution is the process in which a substance forms a. Tablet Dissolution Definition.
From www.researchgate.net
(a) ASD tablet dissolution (GDC0810 dissolved at 60 min) versus XRM Tablet Dissolution Definition Dissolution is the process in which a substance forms a solution. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. The dissolution test in. Tablet Dissolution Definition.
From www.researchgate.net
(A) Dissolution Profile of Immediate Release Core Tablet, (B Tablet Dissolution Definition Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different. Tablet Dissolution Definition.
From www.scribd.com
Theory of Drug Dissolution Dissolution (Chemistry) Solution Tablet Dissolution Definition Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. Dissolution testing measures the extent and rate of solution formation from a dosage form, such as tablet, capsule,. The administration of drugs via oral dosage forms is one of the most common and effective means of delivering. Dissolution testing provides critical information. Tablet Dissolution Definition.
From www.researchgate.net
Dissolution data of coated tablets Download Scientific Diagram Tablet Dissolution Definition In vivo, the disintegration and dissolution test of a tablet or capsule is the first step towards therapeutic effect, and control is essential. Dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for. The dissolution test in a usp drug product monograph helps. Tablet Dissolution Definition.
From www.researchgate.net
Indomethacin tablet dissolution (Form I vs. Form II). Download Tablet Dissolution Definition Dissolution limits for ir, dr and er solid oral dosage form for in vitro purposes, usfda had revealed some guidelines. As a quality control test, the dissolution test is. Dissolution testing is an important physiochemical test for the development of solid oral dosage forms, tablets, and capsules. In vivo, the disintegration and dissolution test of a tablet or capsule is. Tablet Dissolution Definition.