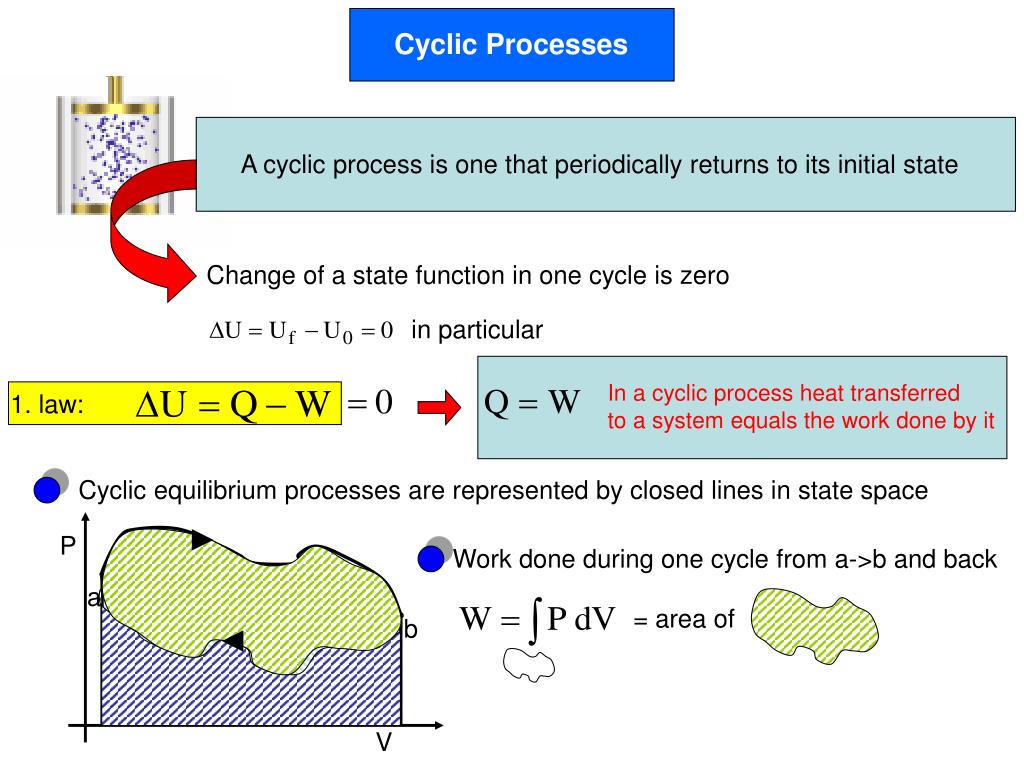
Change In Internal Energy In Cyclic Process . Change in internal energy is given by: In a cyclic transformation the final state of a system is by definition identical to the initial state. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The internal energy change in a cyclic process is zero, and. The overall change of the internal energy. The first law of thermodynamics states that the energy of the universe is. Isothermal expansion, isochoric removal, isothermal. A cyclic process is a sequence of processes that leave the system in the same state as the initial state. Learn how to calculate the net work and heat in a cyclic process with four steps: Where n is the number of moles, f is the degree of freedom of gas, r is.
from www.slideserve.com
Where n is the number of moles, f is the degree of freedom of gas, r is. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Isothermal expansion, isochoric removal, isothermal. Learn how to calculate the net work and heat in a cyclic process with four steps: Change in internal energy is given by: A cyclic process is a sequence of processes that leave the system in the same state as the initial state. In a cyclic transformation the final state of a system is by definition identical to the initial state. The first law of thermodynamics states that the energy of the universe is. The internal energy change in a cyclic process is zero, and. The overall change of the internal energy.
PPT Cyclic Processes PowerPoint Presentation, free download ID2967719
Change In Internal Energy In Cyclic Process A cyclic process is a sequence of processes that leave the system in the same state as the initial state. The first law of thermodynamics states that the energy of the universe is. The overall change of the internal energy. A cyclic process is a sequence of processes that leave the system in the same state as the initial state. Change in internal energy is given by: Where n is the number of moles, f is the degree of freedom of gas, r is. The internal energy change in a cyclic process is zero, and. Learn how to calculate the net work and heat in a cyclic process with four steps: Isothermal expansion, isochoric removal, isothermal. In a cyclic transformation the final state of a system is by definition identical to the initial state. A reaction or process in which heat is transferred to a system from its surroundings is endothermic.
From www.slideserve.com
PPT Thermodynamic Processes PowerPoint Presentation, free download Change In Internal Energy In Cyclic Process Where n is the number of moles, f is the degree of freedom of gas, r is. The first law of thermodynamics states that the energy of the universe is. The overall change of the internal energy. In a cyclic transformation the final state of a system is by definition identical to the initial state. Change in internal energy is. Change In Internal Energy In Cyclic Process.
From www.toppr.com
A gas is taken through the cyclic process described in Figure (a) Find Change In Internal Energy In Cyclic Process Change in internal energy is given by: Learn how to calculate the net work and heat in a cyclic process with four steps: The internal energy change in a cyclic process is zero, and. The overall change of the internal energy. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The first. Change In Internal Energy In Cyclic Process.
From www.youtube.com
A sample of an ideal gas is taken through the cyclic process abca as Change In Internal Energy In Cyclic Process The overall change of the internal energy. Learn how to calculate the net work and heat in a cyclic process with four steps: Isothermal expansion, isochoric removal, isothermal. Where n is the number of moles, f is the degree of freedom of gas, r is. The first law of thermodynamics states that the energy of the universe is. Change in. Change In Internal Energy In Cyclic Process.
From www.tessshebaylo.com
First Law Of Thermodynamics Equation For Cyclic Process Tessshebaylo Change In Internal Energy In Cyclic Process Learn how to calculate the net work and heat in a cyclic process with four steps: Change in internal energy is given by: Where n is the number of moles, f is the degree of freedom of gas, r is. A cyclic process is a sequence of processes that leave the system in the same state as the initial state.. Change In Internal Energy In Cyclic Process.
From www.slideserve.com
PPT Thermodynamic Processes PowerPoint Presentation, free download Change In Internal Energy In Cyclic Process In a cyclic transformation the final state of a system is by definition identical to the initial state. Learn how to calculate the net work and heat in a cyclic process with four steps: The internal energy change in a cyclic process is zero, and. Change in internal energy is given by: The overall change of the internal energy. A. Change In Internal Energy In Cyclic Process.
From www.numerade.com
SOLVED thermodynamic system undergoes cyclic process as shown in the Change In Internal Energy In Cyclic Process Where n is the number of moles, f is the degree of freedom of gas, r is. The first law of thermodynamics states that the energy of the universe is. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. In a cyclic transformation the final state of a system is by definition. Change In Internal Energy In Cyclic Process.
From www.slideserve.com
PPT James Joule and the mechanical equivalent of heat PowerPoint Change In Internal Energy In Cyclic Process Where n is the number of moles, f is the degree of freedom of gas, r is. The overall change of the internal energy. In a cyclic transformation the final state of a system is by definition identical to the initial state. The internal energy change in a cyclic process is zero, and. The first law of thermodynamics states that. Change In Internal Energy In Cyclic Process.
From www.youtube.com
How to Find the Work Done in Cyclic Process in Thermodynamics (Internal Change In Internal Energy In Cyclic Process Change in internal energy is given by: The first law of thermodynamics states that the energy of the universe is. Where n is the number of moles, f is the degree of freedom of gas, r is. In a cyclic transformation the final state of a system is by definition identical to the initial state. The overall change of the. Change In Internal Energy In Cyclic Process.
From www.thebigger.com
Explain Cyclic Process with example. Change In Internal Energy In Cyclic Process Where n is the number of moles, f is the degree of freedom of gas, r is. The overall change of the internal energy. Change in internal energy is given by: In a cyclic transformation the final state of a system is by definition identical to the initial state. The internal energy change in a cyclic process is zero, and.. Change In Internal Energy In Cyclic Process.
From www.doubtnut.com
Explain with diagram stepbystep energy conversion in Thermal powe Change In Internal Energy In Cyclic Process In a cyclic transformation the final state of a system is by definition identical to the initial state. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Change in internal energy is given by: Where n is the number of moles, f is the degree of freedom of gas, r is. The. Change In Internal Energy In Cyclic Process.
From pforphysicss.blogspot.com
Internal EnergyCharacteristics of Internal Energy Change In Internal Energy In Cyclic Process Learn how to calculate the net work and heat in a cyclic process with four steps: The internal energy change in a cyclic process is zero, and. Isothermal expansion, isochoric removal, isothermal. The overall change of the internal energy. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. In a cyclic transformation. Change In Internal Energy In Cyclic Process.
From www.toppr.com
If the work done by the system is 300 Joule when 100 calorie heat is Change In Internal Energy In Cyclic Process Change in internal energy is given by: The internal energy change in a cyclic process is zero, and. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The overall change of the internal energy. Learn how to calculate the net work and heat in a cyclic process with four steps: Isothermal expansion,. Change In Internal Energy In Cyclic Process.
From www.slideserve.com
PPT CHAPTER 16 THERMODYNAMICS PowerPoint Presentation, free Change In Internal Energy In Cyclic Process Where n is the number of moles, f is the degree of freedom of gas, r is. Change in internal energy is given by: Isothermal expansion, isochoric removal, isothermal. A cyclic process is a sequence of processes that leave the system in the same state as the initial state. In a cyclic transformation the final state of a system is. Change In Internal Energy In Cyclic Process.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Change In Internal Energy In Cyclic Process The overall change of the internal energy. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Change in internal energy is given by: The internal energy change in a cyclic process is zero, and. Where n is the number of moles, f is the degree of freedom of gas, r is. A. Change In Internal Energy In Cyclic Process.
From www.pearson.com
Molar Specific Heat for Constant Volume and Constant Pressure Change In Internal Energy In Cyclic Process The first law of thermodynamics states that the energy of the universe is. Change in internal energy is given by: In a cyclic transformation the final state of a system is by definition identical to the initial state. The internal energy change in a cyclic process is zero, and. Isothermal expansion, isochoric removal, isothermal. Learn how to calculate the net. Change In Internal Energy In Cyclic Process.
From www.slideserve.com
PPT Cyclic Processes PowerPoint Presentation, free download ID2967719 Change In Internal Energy In Cyclic Process The first law of thermodynamics states that the energy of the universe is. Learn how to calculate the net work and heat in a cyclic process with four steps: A cyclic process is a sequence of processes that leave the system in the same state as the initial state. The internal energy change in a cyclic process is zero, and.. Change In Internal Energy In Cyclic Process.
From askfilo.com
Change in internal energy in process CA Filo Change In Internal Energy In Cyclic Process Where n is the number of moles, f is the degree of freedom of gas, r is. Learn how to calculate the net work and heat in a cyclic process with four steps: The first law of thermodynamics states that the energy of the universe is. Isothermal expansion, isochoric removal, isothermal. Change in internal energy is given by: In a. Change In Internal Energy In Cyclic Process.
From slideplayer.com
Thermodynamic Processes ppt download Change In Internal Energy In Cyclic Process A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Isothermal expansion, isochoric removal, isothermal. The first law of thermodynamics states that the energy of the universe is. The internal energy change in a cyclic process is zero, and. A cyclic process is a sequence of processes that leave the system in the. Change In Internal Energy In Cyclic Process.
From www.doubtnut.com
Figure shows the variation of internal energy (U) with the pressure (P Change In Internal Energy In Cyclic Process The first law of thermodynamics states that the energy of the universe is. Change in internal energy is given by: Learn how to calculate the net work and heat in a cyclic process with four steps: Isothermal expansion, isochoric removal, isothermal. A cyclic process is a sequence of processes that leave the system in the same state as the initial. Change In Internal Energy In Cyclic Process.
From brainly.com
A gas is taken through the cyclic process described in the figure. (The Change In Internal Energy In Cyclic Process Where n is the number of moles, f is the degree of freedom of gas, r is. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Isothermal expansion, isochoric removal, isothermal. In a cyclic transformation the final state of a system is by definition identical to the initial state. Learn how to. Change In Internal Energy In Cyclic Process.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Change In Internal Energy In Cyclic Process In a cyclic transformation the final state of a system is by definition identical to the initial state. Learn how to calculate the net work and heat in a cyclic process with four steps: A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The internal energy change in a cyclic process is. Change In Internal Energy In Cyclic Process.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Change In Internal Energy In Cyclic Process A reaction or process in which heat is transferred to a system from its surroundings is endothermic. In a cyclic transformation the final state of a system is by definition identical to the initial state. The internal energy change in a cyclic process is zero, and. Learn how to calculate the net work and heat in a cyclic process with. Change In Internal Energy In Cyclic Process.
From www.tessshebaylo.com
What Is The Mathematical Equation For Calculating Internal Energy Of A Change In Internal Energy In Cyclic Process The first law of thermodynamics states that the energy of the universe is. The internal energy change in a cyclic process is zero, and. The overall change of the internal energy. In a cyclic transformation the final state of a system is by definition identical to the initial state. Isothermal expansion, isochoric removal, isothermal. A reaction or process in which. Change In Internal Energy In Cyclic Process.
From www.toppr.com
One mole of an ideal mono atamic gas is taken round the cyclic process Change In Internal Energy In Cyclic Process A cyclic process is a sequence of processes that leave the system in the same state as the initial state. In a cyclic transformation the final state of a system is by definition identical to the initial state. Learn how to calculate the net work and heat in a cyclic process with four steps: A reaction or process in which. Change In Internal Energy In Cyclic Process.
From en.ppt-online.org
Heat flow and the first law of thermodynamics. Kind of thermodynamic Change In Internal Energy In Cyclic Process The first law of thermodynamics states that the energy of the universe is. Isothermal expansion, isochoric removal, isothermal. In a cyclic transformation the final state of a system is by definition identical to the initial state. The overall change of the internal energy. Where n is the number of moles, f is the degree of freedom of gas, r is.. Change In Internal Energy In Cyclic Process.
From www.youtube.com
CHEM 101 Change in Internal Energy, Heat, and PressureVolume Work 2 Change In Internal Energy In Cyclic Process The internal energy change in a cyclic process is zero, and. A cyclic process is a sequence of processes that leave the system in the same state as the initial state. The first law of thermodynamics states that the energy of the universe is. Isothermal expansion, isochoric removal, isothermal. In a cyclic transformation the final state of a system is. Change In Internal Energy In Cyclic Process.
From www.justanswer.com
Goal Solve for the performance coefficient of a refrigerator using a Change In Internal Energy In Cyclic Process A cyclic process is a sequence of processes that leave the system in the same state as the initial state. In a cyclic transformation the final state of a system is by definition identical to the initial state. Where n is the number of moles, f is the degree of freedom of gas, r is. Isothermal expansion, isochoric removal, isothermal.. Change In Internal Energy In Cyclic Process.
From www.youtube.com
The `PV` diagram of a gas undergoing a cyclic process ABCDA is shown Change In Internal Energy In Cyclic Process Learn how to calculate the net work and heat in a cyclic process with four steps: The overall change of the internal energy. A cyclic process is a sequence of processes that leave the system in the same state as the initial state. Isothermal expansion, isochoric removal, isothermal. Where n is the number of moles, f is the degree of. Change In Internal Energy In Cyclic Process.
From www.venkatsacademy.com
Cyclic Process,Reversible Process and Work Done Graphs IIT JEE and Change In Internal Energy In Cyclic Process Where n is the number of moles, f is the degree of freedom of gas, r is. The internal energy change in a cyclic process is zero, and. In a cyclic transformation the final state of a system is by definition identical to the initial state. Learn how to calculate the net work and heat in a cyclic process with. Change In Internal Energy In Cyclic Process.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID4342222 Change In Internal Energy In Cyclic Process The first law of thermodynamics states that the energy of the universe is. Learn how to calculate the net work and heat in a cyclic process with four steps: The internal energy change in a cyclic process is zero, and. Where n is the number of moles, f is the degree of freedom of gas, r is. A reaction or. Change In Internal Energy In Cyclic Process.
From www.slideserve.com
PPT Theory and Thermodynamics PowerPoint Presentation, free Change In Internal Energy In Cyclic Process The internal energy change in a cyclic process is zero, and. In a cyclic transformation the final state of a system is by definition identical to the initial state. Isothermal expansion, isochoric removal, isothermal. The first law of thermodynamics states that the energy of the universe is. A cyclic process is a sequence of processes that leave the system in. Change In Internal Energy In Cyclic Process.
From pforphysicss.blogspot.com
Internal EnergyCharacteristics of Internal Energy Change In Internal Energy In Cyclic Process The overall change of the internal energy. The first law of thermodynamics states that the energy of the universe is. The internal energy change in a cyclic process is zero, and. In a cyclic transformation the final state of a system is by definition identical to the initial state. Where n is the number of moles, f is the degree. Change In Internal Energy In Cyclic Process.
From www.doubtnut.com
In a cyclic process shown in the figure an ideal gas is adiabatically Change In Internal Energy In Cyclic Process Change in internal energy is given by: Learn how to calculate the net work and heat in a cyclic process with four steps: The overall change of the internal energy. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The internal energy change in a cyclic process is zero, and. Where n. Change In Internal Energy In Cyclic Process.
From jamar-kguzman.blogspot.com
An Ideal Gas Is Made to Undergo the Cyclic Process Change In Internal Energy In Cyclic Process In a cyclic transformation the final state of a system is by definition identical to the initial state. The overall change of the internal energy. The first law of thermodynamics states that the energy of the universe is. Isothermal expansion, isochoric removal, isothermal. Learn how to calculate the net work and heat in a cyclic process with four steps: Where. Change In Internal Energy In Cyclic Process.
From quizgecko.com
In an is cyclic process shown in the figure, when the gas is taken from Change In Internal Energy In Cyclic Process A cyclic process is a sequence of processes that leave the system in the same state as the initial state. In a cyclic transformation the final state of a system is by definition identical to the initial state. The first law of thermodynamics states that the energy of the universe is. Change in internal energy is given by: The internal. Change In Internal Energy In Cyclic Process.