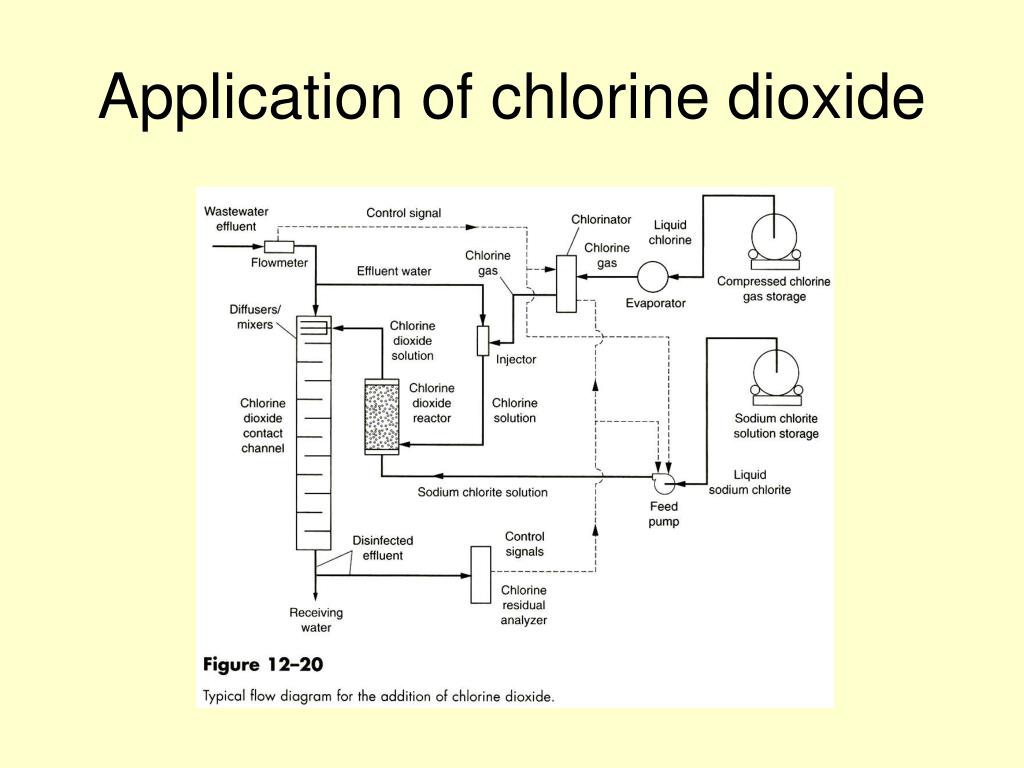
Chlorine Dioxide Ph . It is effective at low concentrations, has less harmful byproducts than chlorine and is. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. If those processes are used, the ph is. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate;
from giotthdcy.blob.core.windows.net
Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. If those processes are used, the ph is. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; It is effective at low concentrations, has less harmful byproducts than chlorine and is. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,.
Disinfection Efficacy Chlorine Dioxide at Mildred Pryor blog
Chlorine Dioxide Ph Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. If those processes are used, the ph is. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. It is effective at low concentrations, has less harmful byproducts than chlorine and is.
From giotthdcy.blob.core.windows.net
Disinfection Efficacy Chlorine Dioxide at Mildred Pryor blog Chlorine Dioxide Ph Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. It is effective at low concentrations, has less harmful byproducts than chlorine and is. Clo2 is most often generated by acidifying a solution of sodium. Chlorine Dioxide Ph.
From dir.indiamart.com
Chlorine Dioxide Powder 100490404 Latest Price, Manufacturers Chlorine Dioxide Ph It is effective at low concentrations, has less harmful byproducts than chlorine and is. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. Clo2 is most often generated by acidifying a solution of sodium. Chlorine Dioxide Ph.
From www.vitasoul.co.za
CDS Chlorine Dioxide Solution VitaSoul Chlorine Dioxide Ph It is effective at low concentrations, has less harmful byproducts than chlorine and is. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. If those processes are used, the ph is. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids. Chlorine Dioxide Ph.
From noshly.com
Chlorine dioxide 926 Noshly Wise eating, made easy. Chlorine Dioxide Ph Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. It is effective at low concentrations, has less harmful byproducts than chlorine and is. If those processes are used, the. Chlorine Dioxide Ph.
From commons.wikimedia.org
FileChlorine dioxide solution.jpg Chlorine Dioxide Ph It is effective at low concentrations, has less harmful byproducts than chlorine and is. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine. Chlorine Dioxide Ph.
From www.sheltonmart.com.my
Chlorine Dioxide Precursor (SANICARE SD14) Shelton Mart Office Chlorine Dioxide Ph It is effective at low concentrations, has less harmful byproducts than chlorine and is. If those processes are used, the ph is. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Chlorine dioxide is an oxidizing biocide which does not. Chlorine Dioxide Ph.
From keramikkuminimalis.blogspot.com
10+ Populer Chlorine Disinfectant Or Antiseptic Chlorine Dioxide Ph Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; It is effective at low concentrations, has less harmful byproducts than chlorine and is. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection. Chlorine Dioxide Ph.
From www.slideserve.com
PPT Control Measures for Infectious Diseases PowerPoint Presentation Chlorine Dioxide Ph Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. If those processes are used, the ph is. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; It is effective at low concentrations, has less harmful byproducts than chlorine. Chlorine Dioxide Ph.
From chlorinedioxidesolution.ca
Chlorine Dioxide Formula chlorinedioxide Chlorine Dioxide Ph Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. It is effective at low concentrations, has less harmful. Chlorine Dioxide Ph.
From www.youtube.com
The Multiple Uses of Chlorine Dioxide CLO2 YouTube Chlorine Dioxide Ph Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; It is effective at low concentrations, has less harmful byproducts than chlorine and is. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. If those processes are used, the. Chlorine Dioxide Ph.
From shopee.ph
Organiks Chlorine Dioxide Multi Purpose Disinfectant Shopee Philippines Chlorine Dioxide Ph Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. It is effective at low concentrations, has less harmful byproducts than chlorine and is. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. If those processes are used, the ph is. Clo2 is most. Chlorine Dioxide Ph.
From www.indiamart.com
Stabilized Chlorine Dioxide Solution at Rs 200/kg Liquid Chlorine Chlorine Dioxide Ph Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. If those processes are used, the ph is. It is effective at low concentrations, has less harmful byproducts than chlorine and is. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in. Chlorine Dioxide Ph.
From www.researchgate.net
Effect of pH on AOX formation. (a) Reaction of VA with chlorine Chlorine Dioxide Ph Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. If those processes are used, the ph is. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not. Chlorine Dioxide Ph.
From shopee.ph
300g Chlorine Dioxide Tablets Multifunction Swimming Pool Tub Spa Chlorine Dioxide Ph It is effective at low concentrations, has less harmful byproducts than chlorine and is. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. If those processes are used, the ph is. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Clo2 is most often generated by. Chlorine Dioxide Ph.
From mastermineralsolutions.com
Disinfectant Chlorine Dioxide MMS Master Mineral Solutions Chlorine Dioxide Ph Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. If those processes are used, the ph is. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. Chlorine dioxide. Chlorine Dioxide Ph.
From miracleproducts.shop
CDS Chlorine Dioxide Miracle Products Chlorine Dioxide Ph Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. If those processes are used, the ph is. Clo2 is most often generated. Chlorine Dioxide Ph.
From www.ubuy.com.om
Buy The Original Chlorine Dioxide Kit 2 Part Classic Liquid 11 Set Chlorine Dioxide Ph Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. It is effective at low concentrations, has less harmful byproducts than chlorine and is. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in. Chlorine Dioxide Ph.
From bartovation.com
Chlorine Dioxide Single Factor Test Strips, 010 ppm Chlorine Dioxide Ph Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. It is. Chlorine Dioxide Ph.
From www.processinstruments.net
24 Turbidity, Chlorine Dioxide and pH Systems for China, All With Chlorine Dioxide Ph Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. It is effective at low concentrations, has less harmful byproducts than chlorine and is. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls.. Chlorine Dioxide Ph.
From hoachatdackhang.com
[Bán] Hóa Chất Chlorine Dioxide Giá Tốt, Uy Tín Chlorine Dioxide Ph Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. Chlorine dioxide removes biofilms and. Chlorine Dioxide Ph.
From giotthdcy.blob.core.windows.net
Disinfection Efficacy Chlorine Dioxide at Mildred Pryor blog Chlorine Dioxide Ph Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. If those processes are used, the ph is. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; It is effective at low concentrations, has less harmful byproducts than chlorine and is. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for. Chlorine Dioxide Ph.
From www.lovibond.com
Test the chlorine dioxide level with the perfect fit Drop Test Kit Chlorine Dioxide Ph Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. It is effective at low concentrations, has less. Chlorine Dioxide Ph.
From www.linkedin.com
Disinfection Mechanism of Chlorine Dioxide (CIO2) Chlorine Dioxide Ph Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; If those processes are used, the ph is. It is effective at low concentrations, has less harmful byproducts than chlorine and is. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not. Chlorine Dioxide Ph.
From www.indiamart.com
pH And Chlorine Dioxide PTM 900 at best price in Thane by Amkette Chlorine Dioxide Ph Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. If those processes are used, the ph is. Clo2 is most often generated by. Chlorine Dioxide Ph.
From www.galleon.ph
Chlorine Dioxide Kit 2 Part Liquid Classic 11 Set Hydrochloric Acid Chlorine Dioxide Ph Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of. Chlorine Dioxide Ph.
From giotthdcy.blob.core.windows.net
Disinfection Efficacy Chlorine Dioxide at Mildred Pryor blog Chlorine Dioxide Ph Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. It is effective at low concentrations, has less harmful byproducts than chlorine and is. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; If those processes are used,. Chlorine Dioxide Ph.
From www.lazada.com.ph
Water quality detection reagent PH residual chlorine total chlorine Chlorine Dioxide Ph Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. It is effective at low concentrations, has less harmful. Chlorine Dioxide Ph.
From perfectlyhealthy.com
Chlorine Dioxide (Cl02) Perfectly Healthy Chlorine Dioxide Ph Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. It is effective at low concentrations, has less harmful byproducts than chlorine and is. If those processes are used, the ph is. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Moreover, in contrast to chlorine, chlorine dioxide's efficiency for. Chlorine Dioxide Ph.
From www.indiamart.com
Chlorine Dioxide Powder at Rs 350/kg 100490404, क्लोरीन डाइऑक्साइड Chlorine Dioxide Ph Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. It is effective at low concentrations, has less harmful byproducts than chlorine and is. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. Chlorine dioxide is an oxidizing biocide which does not. Chlorine Dioxide Ph.
From blog.orendatech.com
Understanding Breakpoint Chlorination Chlorine Dioxide Ph If those processes are used, the ph is. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Chlorine. Chlorine Dioxide Ph.
From www.researchgate.net
(PDF) Improving chlorine dioxide bleaching efficiency by selecting the Chlorine Dioxide Ph It is effective at low concentrations, has less harmful byproducts than chlorine and is. Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Furthermore, the oxidation and disinfection. Chlorine Dioxide Ph.
From shopee.ph
Chlorine Dioxide Colorimeter Chlorine Dioxide Concentration Chlorine Dioxide Ph If those processes are used, the ph is. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. It is effective at low concentrations, has less harmful byproducts than chlorine and is. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not. Chlorine Dioxide Ph.
From www.phwatertechnologies.co.uk
Chlorine Dioxide ph Water Technologies Chlorine Dioxide Ph Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; If those processes are used, the ph is. It is effective at low concentrations, has less harmful byproducts than chlorine and is. Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids. Chlorine Dioxide Ph.
From eaiwater.com
Chlorine Dioxide Generators Chlorine Dioxide Ph Furthermore, the oxidation and disinfection capabilities of clo2 are independent of the ph of. Clo2 is most often generated by acidifying a solution of sodium chlorite or sodium chlorate; Chlorine dioxide removes biofilms and inactivates germs by destroying cell walls. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine. Chlorine Dioxide Ph.
From www.pyxis-lab.com
OXIPANEL™ IK765SS Free Chlorine or Chlorine Dioxide Panel Chlorine Dioxide Ph Moreover, in contrast to chlorine, chlorine dioxide's efficiency for disinfection does not vary with ph or in the presence of ammonia, and it does not oxidize. Chlorine dioxide is an oxidizing biocide which does not form hydrochlorous acids in water, but instead exists as dissolved chlorine dioxide,. Clo2 is most often generated by acidifying a solution of sodium chlorite or. Chlorine Dioxide Ph.