Is Acetone More Polar Than Ethyl Acetate . Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Commonly used in 0 to 30% concentrations. The simplest and most prominent example is. ethyl acetate more polar than hexane, but not so much that it is miscible with water. Common solvents arranged from the least polar to the most polar there is only little correlation for a molecule to have dipole moment and being polar. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. 1) ethyl acetate/hexane: However, removing the solvent completely on a rotary.
from www.youtube.com
there is only little correlation for a molecule to have dipole moment and being polar. Commonly used in 0 to 30% concentrations. Common solvents arranged from the least polar to the most polar burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. 1) ethyl acetate/hexane: However, removing the solvent completely on a rotary. ethyl acetate more polar than hexane, but not so much that it is miscible with water. The simplest and most prominent example is. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline.
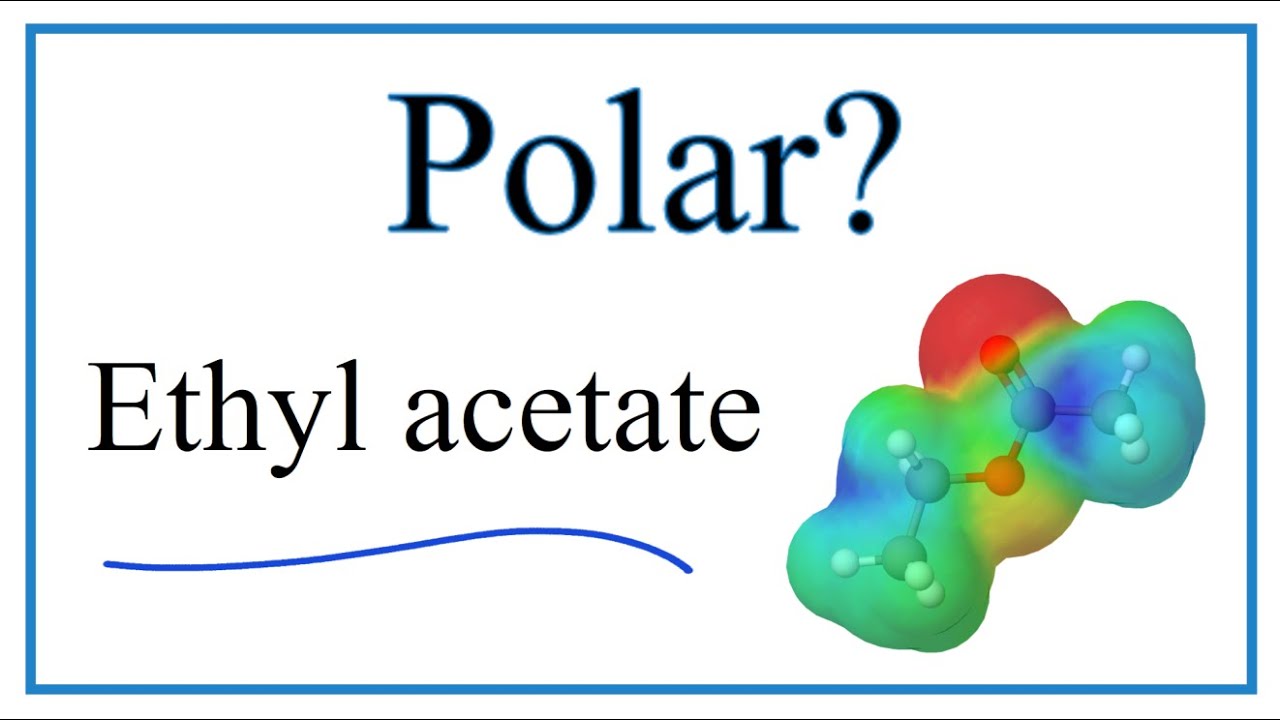
Is Ethyl acetate (C4H8O2) Polar or NonPolar YouTube
Is Acetone More Polar Than Ethyl Acetate Common solvents arranged from the least polar to the most polar ethyl acetate more polar than hexane, but not so much that it is miscible with water. there is only little correlation for a molecule to have dipole moment and being polar. However, removing the solvent completely on a rotary. Commonly used in 0 to 30% concentrations. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Common solvents arranged from the least polar to the most polar burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. 1) ethyl acetate/hexane: The simplest and most prominent example is.
From www.coursehero.com
[Solved] answer all the questions Part III. Polarity (Bond polarity Is Acetone More Polar Than Ethyl Acetate Commonly used in 0 to 30% concentrations. Common solvents arranged from the least polar to the most polar Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. 1) ethyl acetate/hexane: there is only little correlation for a molecule to have dipole moment and being polar. However, removing the solvent completely on a rotary. burdick. Is Acetone More Polar Than Ethyl Acetate.
From chemistry.stackexchange.com
organic chemistry Why bond energy of acetone is more though it is Is Acetone More Polar Than Ethyl Acetate Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Commonly used in 0 to 30% concentrations. there is only little correlation for a molecule to have dipole moment and being polar. ethyl acetate more polar than hexane, but not so much that it is miscible with water. However, removing the solvent completely on a rotary.. Is Acetone More Polar Than Ethyl Acetate.
From www.alamy.com
Ethyl acetate, ethyl ethanoate molecule. It is acetate ester, polar Is Acetone More Polar Than Ethyl Acetate ethyl acetate more polar than hexane, but not so much that it is miscible with water. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. However, removing the solvent completely on a rotary. . Is Acetone More Polar Than Ethyl Acetate.
From www.researchgate.net
Why we use ACETONE in "acetone insolubility" study instead of other Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. The simplest and most prominent example is. 1) ethyl acetate/hexane: there is only little correlation for a molecule to have dipole moment and being. Is Acetone More Polar Than Ethyl Acetate.
From chem-net.blogspot.com
Solvent Effects and SN2 and SN1 reactions Nucleophilic Substitution Is Acetone More Polar Than Ethyl Acetate However, removing the solvent completely on a rotary. Common solvents arranged from the least polar to the most polar ethyl acetate more polar than hexane, but not so much that it is miscible with water. 1) ethyl acetate/hexane: there is only little correlation for a molecule to have dipole moment and being polar. burdick & jackson. Is Acetone More Polar Than Ethyl Acetate.
From dxouiylma.blob.core.windows.net
Is Acetone More Polar Than Propanol at Kayla Thomas blog Is Acetone More Polar Than Ethyl Acetate Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. However, removing the solvent completely on a rotary. The simplest and most prominent example is. there is only little correlation for a molecule to have dipole moment and being polar. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it. Is Acetone More Polar Than Ethyl Acetate.
From courses.lumenlearning.com
1.8. Intermolecular forces Organic Chemistry 1 An open textbook Is Acetone More Polar Than Ethyl Acetate ethyl acetate more polar than hexane, but not so much that it is miscible with water. Commonly used in 0 to 30% concentrations. 1) ethyl acetate/hexane: The simplest and most prominent example is. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Common solvents arranged from the least polar to the most polar However, removing. Is Acetone More Polar Than Ethyl Acetate.
From www.researchgate.net
Absorbance values of ethyl acetate, acetone and methanol extracts of Is Acetone More Polar Than Ethyl Acetate Common solvents arranged from the least polar to the most polar The simplest and most prominent example is. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. ethyl acetate more polar than hexane, but not so much that it is miscible with water. Commonly used in. Is Acetone More Polar Than Ethyl Acetate.
From www.youtube.com
Is ethyl acetate polar or nonpolar solvent? YouTube Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. 1) ethyl acetate/hexane: ethyl acetate more polar than hexane, but not so much that it is miscible with water. Common solvents arranged from the. Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED Which of these TLC solvent systems listed below is the most Is Acetone More Polar Than Ethyl Acetate The simplest and most prominent example is. 1) ethyl acetate/hexane: Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Commonly used in 0 to 30% concentrations. there is only little correlation for a molecule to have dipole moment and being polar. burdick & jackson solvents are arranged in order of increasing polarity index, a. Is Acetone More Polar Than Ethyl Acetate.
From www.coursehero.com
[Solved] Indicate the most polar solvent. a. Ethyl Acetate b. Hexane Is Acetone More Polar Than Ethyl Acetate there is only little correlation for a molecule to have dipole moment and being polar. The simplest and most prominent example is. 1) ethyl acetate/hexane: Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. ethyl acetate more polar than hexane, but not so much that it is miscible with water. However, removing the solvent. Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED Which among the following solvents is a) least polar b) most Is Acetone More Polar Than Ethyl Acetate However, removing the solvent completely on a rotary. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Commonly used in 0 to 30% concentrations. Common solvents arranged from the least polar to the most polar there is only little correlation for a molecule to have dipole moment and being polar. 1) ethyl acetate/hexane: ethyl. Is Acetone More Polar Than Ethyl Acetate.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone More Polar Than Ethyl Acetate The simplest and most prominent example is. burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. there is only little correlation for a molecule to have dipole moment and being polar. 1) ethyl acetate/hexane: Commonly used in 0 to 30% concentrations. Water acetic acid. Is Acetone More Polar Than Ethyl Acetate.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps Is Acetone More Polar Than Ethyl Acetate Common solvents arranged from the least polar to the most polar Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. The simplest and most prominent example is. However, removing the solvent completely on a rotary. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not.. Is Acetone More Polar Than Ethyl Acetate.
From in.pinterest.com
Is Ethyl Acetate Polar or NonPolar? (C4H8O2) Ethyl acetate Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. there is only little correlation for a molecule to have dipole moment and being polar. However, removing the solvent completely on a rotary. Common solvents arranged from the least polar to the most polar The simplest and. Is Acetone More Polar Than Ethyl Acetate.
From dxouiylma.blob.core.windows.net
Is Acetone More Polar Than Propanol at Kayla Thomas blog Is Acetone More Polar Than Ethyl Acetate Commonly used in 0 to 30% concentrations. burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. there is only little correlation for a molecule to have dipole moment and being polar. The simplest. Is Acetone More Polar Than Ethyl Acetate.
From dxodhvelq.blob.core.windows.net
Is Ether Acetone Polar at Bonnie Jimenez blog Is Acetone More Polar Than Ethyl Acetate However, removing the solvent completely on a rotary. burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. there is only little correlation for a molecule to have dipole moment and being polar. 23 rows however, acetone is still considered a polar aprotic solvent, despite. Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED Table Relative polarity of common mobile phases Acetic acid Is Acetone More Polar Than Ethyl Acetate there is only little correlation for a molecule to have dipole moment and being polar. However, removing the solvent completely on a rotary. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. The simplest and most prominent example is. ethyl acetate more polar than hexane,. Is Acetone More Polar Than Ethyl Acetate.
From www.youtube.com
Is C3H6O (Acetone) Polar or NonPolar? YouTube Is Acetone More Polar Than Ethyl Acetate 1) ethyl acetate/hexane: Commonly used in 0 to 30% concentrations. However, removing the solvent completely on a rotary. The simplest and most prominent example is. Common solvents arranged from the least polar to the most polar ethyl acetate more polar than hexane, but not so much that it is miscible with water. there is only little correlation. Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED 13 14 15 16 Ethyl acetate is polar and isoamyl acetate is Is Is Acetone More Polar Than Ethyl Acetate However, removing the solvent completely on a rotary. 1) ethyl acetate/hexane: there is only little correlation for a molecule to have dipole moment and being polar. ethyl acetate more polar than hexane, but not so much that it is miscible with water. Common solvents arranged from the least polar to the most polar Commonly used in 0. Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED7 Consider the followingtwo molecules Et0SCF3 Et0sCH3 Is Acetone More Polar Than Ethyl Acetate However, removing the solvent completely on a rotary. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. Water acetic acid ethyleneglycol methanol ethanol isopropanol. Is Acetone More Polar Than Ethyl Acetate.
From www.differencebetween.com
Difference Between Acetone and Acetate Compare the Difference Between Is Acetone More Polar Than Ethyl Acetate Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. However, removing the solvent completely on a rotary. Common solvents arranged from the least polar to the most polar 1) ethyl acetate/hexane: The simplest and most prominent example is. ethyl acetate more polar than hexane, but not so much that it is miscible with water. Commonly. Is Acetone More Polar Than Ethyl Acetate.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Master Is Acetone More Polar Than Ethyl Acetate The simplest and most prominent example is. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. However, removing the solvent completely on a rotary. Commonly used in 0 to 30% concentrations. burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. 23 rows. Is Acetone More Polar Than Ethyl Acetate.
From www.slideshare.net
Solvents used in pharmacy Is Acetone More Polar Than Ethyl Acetate 1) ethyl acetate/hexane: The simplest and most prominent example is. burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. However, removing the solvent. Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED Rank hexane, water, ethanol, acetone, and ethyl acetate from Is Acetone More Polar Than Ethyl Acetate Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Common solvents arranged from the least polar to the most polar ethyl acetate more polar than hexane, but not so much that it is miscible with water. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. Is Acetone More Polar Than Ethyl Acetate.
From www.alamy.com
Ethyl acetate, ethyl ethanoate molecule. It is acetate ester, polar Is Acetone More Polar Than Ethyl Acetate However, removing the solvent completely on a rotary. The simplest and most prominent example is. 1) ethyl acetate/hexane: Common solvents arranged from the least polar to the most polar there is only little correlation for a molecule to have dipole moment and being polar. burdick & jackson solvents are arranged in order of increasing polarity index, a. Is Acetone More Polar Than Ethyl Acetate.
From www.caymanchem.com
Solubility Rules Chart.png Is Acetone More Polar Than Ethyl Acetate Commonly used in 0 to 30% concentrations. 1) ethyl acetate/hexane: Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. However, removing the solvent completely on a rotary. there is only little correlation for a molecule to have dipole moment and being polar. ethyl acetate more polar than hexane, but not so much that it. Is Acetone More Polar Than Ethyl Acetate.
From www.nagwa.com
Question Video Determining Whether Some Common Simple Molecular Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. The simplest and most prominent example is. However, removing the solvent completely on a rotary. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. burdick & jackson solvents are arranged in order of increasing. Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED Q2) List the following compounds from least polar to most polar Is Acetone More Polar Than Ethyl Acetate The simplest and most prominent example is. there is only little correlation for a molecule to have dipole moment and being polar. Commonly used in 0 to 30% concentrations. However, removing the solvent completely on a rotary. burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of. Is Acetone More Polar Than Ethyl Acetate.
From www.youtube.com
Is Ethyl acetate (C4H8O2) Polar or NonPolar YouTube Is Acetone More Polar Than Ethyl Acetate Commonly used in 0 to 30% concentrations. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Common solvents arranged from the least polar to the most polar However, removing the solvent completely on a rotary. ethyl acetate more polar than hexane, but not so much that. Is Acetone More Polar Than Ethyl Acetate.
From studylib.net
Acetone as an Alternative to Ethyl Acetate Is Acetone More Polar Than Ethyl Acetate 1) ethyl acetate/hexane: Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Common solvents arranged from the least polar to the most polar burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. The simplest and most prominent example is. there is. Is Acetone More Polar Than Ethyl Acetate.
From courses.lumenlearning.com
Properties of Liquids Chemistry for Majors Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. ethyl acetate more polar than hexane, but not so much that it is miscible with water. The simplest and most prominent example is. Commonly used in 0 to 30% concentrations. 1) ethyl acetate/hexane: burdick &. Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVEDTLC analysis of un unknown mixture on silica gel plate was Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Commonly used in 0 to 30% concentrations. there is only little correlation for a molecule to have dipole moment and being polar. burdick & jackson solvents are arranged in order of increasing polarity index, a relative. Is Acetone More Polar Than Ethyl Acetate.
From dxouiylma.blob.core.windows.net
Is Acetone More Polar Than Propanol at Kayla Thomas blog Is Acetone More Polar Than Ethyl Acetate Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. there is only little correlation for a molecule to have dipole moment and being polar. Common solvents arranged from the least polar to the most polar However, removing the solvent completely on a rotary. Commonly used in 0 to 30% concentrations. burdick & jackson solvents are. Is Acetone More Polar Than Ethyl Acetate.
From www.researchgate.net
Polarizability spectra for water, acetone, and ethanol. Each of the Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the. However, removing the solvent completely on a rotary. there is only little correlation for. Is Acetone More Polar Than Ethyl Acetate.