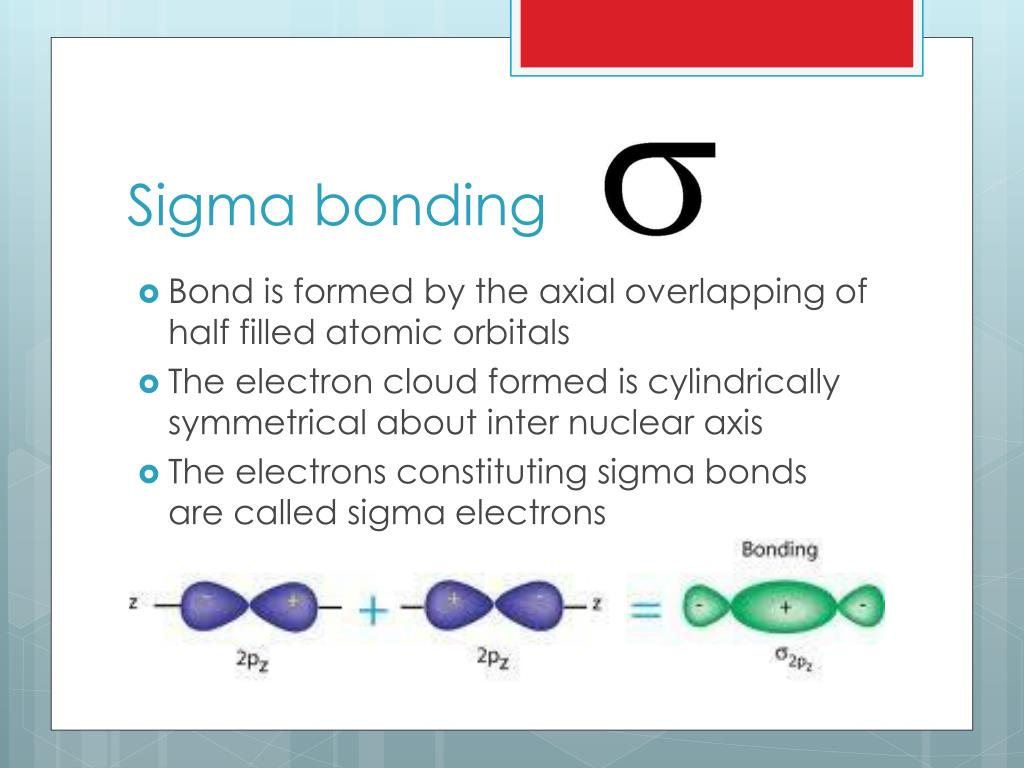
Pi Vs Sigma Bond . Axial overlapping of two orbitals forms sigma bond while lateral. Although the difference between sigma and pi bond are complementary with each other. It is obvious that these two bonds are very important with the many differences. Together they make the c=c double bond. the main difference between sigma bond and pi bond is their formation; Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. basics of sigma and pi bonds. sigma and pi bonds are formed by the overlap of atomic orbitals. Still, some more important points are significant as follows:
from www.slideserve.com
Together they make the c=c double bond. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Still, some more important points are significant as follows: It is obvious that these two bonds are very important with the many differences. Although the difference between sigma and pi bond are complementary with each other. Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. Axial overlapping of two orbitals forms sigma bond while lateral. sigma and pi bonds are formed by the overlap of atomic orbitals. basics of sigma and pi bonds. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals.
PPT Sigma and Pi Bonding PowerPoint Presentation, free download ID
Pi Vs Sigma Bond basics of sigma and pi bonds. Still, some more important points are significant as follows: Although the difference between sigma and pi bond are complementary with each other. basics of sigma and pi bonds. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Axial overlapping of two orbitals forms sigma bond while lateral. Together they make the c=c double bond. sigma and pi bonds are formed by the overlap of atomic orbitals. It is obvious that these two bonds are very important with the many differences. Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. the main difference between sigma bond and pi bond is their formation;
From www.coursehero.com
[Solved] sketch sigma and pi bond from p orbital Course Hero Pi Vs Sigma Bond the main difference between sigma bond and pi bond is their formation; It is obvious that these two bonds are very important with the many differences. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. basics of sigma and pi bonds. Together they make the c=c. Pi Vs Sigma Bond.
From www.slideserve.com
PPT Hybridization The Blending of Orbitals PowerPoint Presentation Pi Vs Sigma Bond Axial overlapping of two orbitals forms sigma bond while lateral. Although the difference between sigma and pi bond are complementary with each other. basics of sigma and pi bonds. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Sigma and pi bonds are an aspect of valence bond theory and. Pi Vs Sigma Bond.
From www.youtube.com
Sigma and Pi Bonding YouTube Pi Vs Sigma Bond Together they make the c=c double bond. Axial overlapping of two orbitals forms sigma bond while lateral. Still, some more important points are significant as follows: It is obvious that these two bonds are very important with the many differences. basics of sigma and pi bonds. the main difference between sigma bond and pi bond is their formation;. Pi Vs Sigma Bond.
From alvarroskun.blogspot.com
Pi Bond And Sigma Bond How to count sigma and pi bonds Quora 3 Pi Vs Sigma Bond basics of sigma and pi bonds. the main difference between sigma bond and pi bond is their formation; Although the difference between sigma and pi bond are complementary with each other. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Axial overlapping of two orbitals forms. Pi Vs Sigma Bond.
From chemisfast.blogspot.com
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY Pi Vs Sigma Bond Still, some more important points are significant as follows: the main difference between sigma bond and pi bond is their formation; sigma and pi bonds are formed by the overlap of atomic orbitals. It is obvious that these two bonds are very important with the many differences. Although the difference between sigma and pi bond are complementary with. Pi Vs Sigma Bond.
From pediaa.com
Difference Between Sigma and Pi Bond Definition, Characteristics Pi Vs Sigma Bond It is obvious that these two bonds are very important with the many differences. the main difference between sigma bond and pi bond is their formation; Still, some more important points are significant as follows: Together they make the c=c double bond. Axial overlapping of two orbitals forms sigma bond while lateral. sigma and pi bonds are types. Pi Vs Sigma Bond.
From www.meritnation.com
Distinguish between sigma and a pi bond Chemistry Chemical Bonding Pi Vs Sigma Bond sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Although the difference between sigma and pi bond are complementary with each other. basics of sigma and pi bonds. Still, some more important points are significant as follows: sigma and pi bonds are formed by the overlap of atomic orbitals.. Pi Vs Sigma Bond.
From whyvector.weebly.com
Sigma and pi bonds whyvector Pi Vs Sigma Bond the main difference between sigma bond and pi bond is their formation; basics of sigma and pi bonds. It is obvious that these two bonds are very important with the many differences. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Sigma and pi bonds are. Pi Vs Sigma Bond.
From www.youtube.com
How Many Sigma and Pi Bond (Count Number of Sigma and Pi Bonds) Example Pi Vs Sigma Bond the main difference between sigma bond and pi bond is their formation; Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. basics of sigma and pi bonds. Together they make the c=c double bond. Although the difference between sigma and pi bond are complementary with each other. sigma and. Pi Vs Sigma Bond.
From www.youtube.com
What are sigma and pi bonds YouTube Pi Vs Sigma Bond Together they make the c=c double bond. Still, some more important points are significant as follows: Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. Although the difference between sigma and pi bond are complementary with each other. the main difference between sigma bond and pi bond is their formation; It. Pi Vs Sigma Bond.
From www.youtube.com
How To Calculate Sigma And Pi Bond Super Trick to Find Sigma and Pi Pi Vs Sigma Bond Still, some more important points are significant as follows: sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. basics of sigma and pi bonds. sigma and pi bonds are formed by the overlap of atomic orbitals. It is obvious that these two bonds are very important with the many. Pi Vs Sigma Bond.
From present5.com
3 Lecture Chemical bonding and Molecular Structure Pi Vs Sigma Bond It is obvious that these two bonds are very important with the many differences. Together they make the c=c double bond. Although the difference between sigma and pi bond are complementary with each other. the main difference between sigma bond and pi bond is their formation; Axial overlapping of two orbitals forms sigma bond while lateral. sigma and. Pi Vs Sigma Bond.
From www.difference101.com
Pi vs. Sigma Bond 6 Key Differences, Pros & Cons, Similarities Pi Vs Sigma Bond Although the difference between sigma and pi bond are complementary with each other. Together they make the c=c double bond. It is obvious that these two bonds are very important with the many differences. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Sigma and pi bonds are. Pi Vs Sigma Bond.
From www.sliderbase.com
Sigma and Pi Bonds Pi Vs Sigma Bond sigma and pi bonds are formed by the overlap of atomic orbitals. basics of sigma and pi bonds. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. It is obvious that these two bonds are very important with the many differences. Still, some more important points. Pi Vs Sigma Bond.
From www.youtube.com
What’s the difference between sigma and pi bonds YouTube Pi Vs Sigma Bond It is obvious that these two bonds are very important with the many differences. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Axial overlapping of two orbitals forms sigma bond while lateral. Together they make the c=c double bond. sigma and pi bonds are formed by the overlap of. Pi Vs Sigma Bond.
From ar.inspiredpencil.com
Sigma And Pi Bonds Explained Pi Vs Sigma Bond It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. Although the difference between sigma and pi bond are complementary with each other. It is obvious that these two bonds are very. Pi Vs Sigma Bond.
From www.youtube.com
Differences Between Sigma and Pi bonds YouTube Pi Vs Sigma Bond Still, some more important points are significant as follows: Although the difference between sigma and pi bond are complementary with each other. sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. sigma and pi bonds are types of. Pi Vs Sigma Bond.
From www.ck12.org
Sigma and Pi Bonds CK12 Foundation Pi Vs Sigma Bond basics of sigma and pi bonds. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. It is obvious that these two bonds are very important with the many differences. the main difference between sigma bond and pi bond is their formation; Axial overlapping of two orbitals forms sigma bond. Pi Vs Sigma Bond.
From askanydifference.com
Sigma Bond vs Pi Bond Difference and Comparison Pi Vs Sigma Bond Axial overlapping of two orbitals forms sigma bond while lateral. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Still, some more important points are significant as follows: Together they make the c=c double bond. It is obvious that these two bonds are very important with the many. Pi Vs Sigma Bond.
From vivadifferences.com
12 Difference Between Pi Bond And Sigma Bond With Examples Viva Pi Vs Sigma Bond Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. It is obvious that these two bonds are very important with the many differences. sigma and pi bonds are formed by the overlap of atomic orbitals. Together they make the c=c double bond. Although the difference between sigma and pi bond are. Pi Vs Sigma Bond.
From brainly.in
what is the difference between sigma bond and pi bond Brainly.in Pi Vs Sigma Bond Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. Although the difference between sigma and pi bond are complementary with each other. Together they make the c=c double bond. sigma and pi bonds are formed by the overlap of atomic orbitals. the main difference between sigma bond and pi bond. Pi Vs Sigma Bond.
From www.chemistrylearner.com
Physical Chemistry Chemistry Learner Pi Vs Sigma Bond It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Although the difference between sigma and pi bond are complementary with each other. Axial overlapping of two orbitals forms sigma bond while lateral. It is obvious that these two bonds are very important with the many differences. Sigma and. Pi Vs Sigma Bond.
From www.slideserve.com
PPT Sigma and Pi Bonding PowerPoint Presentation, free download ID Pi Vs Sigma Bond It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Although the difference between sigma and pi bond are complementary with each other. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. sigma and pi bonds are formed by. Pi Vs Sigma Bond.
From pediaa.com
Difference Between Sigma and Pi Bond Definition, Characteristics Pi Vs Sigma Bond the main difference between sigma bond and pi bond is their formation; Together they make the c=c double bond. Axial overlapping of two orbitals forms sigma bond while lateral. sigma and pi bonds are formed by the overlap of atomic orbitals. Still, some more important points are significant as follows: Sigma and pi bonds are an aspect of. Pi Vs Sigma Bond.
From brilliant.org
Sigma and Pi Bonds Brilliant Math & Science Wiki Pi Vs Sigma Bond basics of sigma and pi bonds. Together they make the c=c double bond. Although the difference between sigma and pi bond are complementary with each other. It is obvious that these two bonds are very important with the many differences. Axial overlapping of two orbitals forms sigma bond while lateral. sigma and pi bonds are types of covalent. Pi Vs Sigma Bond.
From www.javatpoint.com
Difference Between Sigma Bond and Pi Bond javatpoint Pi Vs Sigma Bond the main difference between sigma bond and pi bond is their formation; It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Still, some more important points are significant as follows: Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains.. Pi Vs Sigma Bond.
From www.slideserve.com
PPT Hybridization The Blending of Orbitals PowerPoint Presentation Pi Vs Sigma Bond It is obvious that these two bonds are very important with the many differences. basics of sigma and pi bonds. Axial overlapping of two orbitals forms sigma bond while lateral. Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. the main difference between sigma bond and pi bond is their. Pi Vs Sigma Bond.
From www.cloudizsexy.com
Pi Vs Sigma Bond 6 Key Differences Pros And Cons Similarities Free Pi Vs Sigma Bond the main difference between sigma bond and pi bond is their formation; It is obvious that these two bonds are very important with the many differences. basics of sigma and pi bonds. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Axial overlapping of two orbitals. Pi Vs Sigma Bond.
From www.vedantu.com
JEE Calculation of Sigma and Pi Bonds Important Concepts and Tips Pi Vs Sigma Bond It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. the main difference between sigma bond and pi bond is their formation; basics of sigma and pi bonds. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Together. Pi Vs Sigma Bond.
From cloudshareinfo.blogspot.com
How To Count Sigma And Pi Bonds In Benzene cloudshareinfo Pi Vs Sigma Bond It is obvious that these two bonds are very important with the many differences. basics of sigma and pi bonds. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals.. Pi Vs Sigma Bond.
From www.slideserve.com
PPT Sigma ( s ) and pi ( π ) bonding in C 2 H 4 PowerPoint Pi Vs Sigma Bond the main difference between sigma bond and pi bond is their formation; basics of sigma and pi bonds. Although the difference between sigma and pi bond are complementary with each other. It is obvious that these two bonds are very important with the many differences. sigma and pi bonds are formed by the overlap of atomic orbitals.. Pi Vs Sigma Bond.
From www.expii.com
Sigma and Pi Bonds — Definition & Overview Expii Pi Vs Sigma Bond Axial overlapping of two orbitals forms sigma bond while lateral. Although the difference between sigma and pi bond are complementary with each other. sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. the main difference between sigma bond. Pi Vs Sigma Bond.
From alvarroskun.blogspot.com
Pi Bond And Sigma Bond How to count sigma and pi bonds Quora 3 Pi Vs Sigma Bond basics of sigma and pi bonds. It is a fact that, if there is a single bond between two atoms, then this bond will be sigma bond. Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. Although the difference between sigma and pi bond are complementary with each other. the. Pi Vs Sigma Bond.
From www.youtube.com
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry YouTube Pi Vs Sigma Bond Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. basics of sigma and pi bonds. Together they make the c=c double bond. Still, some more important points are significant as follows: Axial overlapping of two orbitals forms sigma bond while lateral. the main difference between sigma bond and pi bond. Pi Vs Sigma Bond.
From www.vedantu.com
How many sigma and pi bonds are there in the given molecule?\\[H C Pi Vs Sigma Bond Although the difference between sigma and pi bond are complementary with each other. It is obvious that these two bonds are very important with the many differences. Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains. the main difference between sigma bond and pi bond is their formation; sigma and. Pi Vs Sigma Bond.