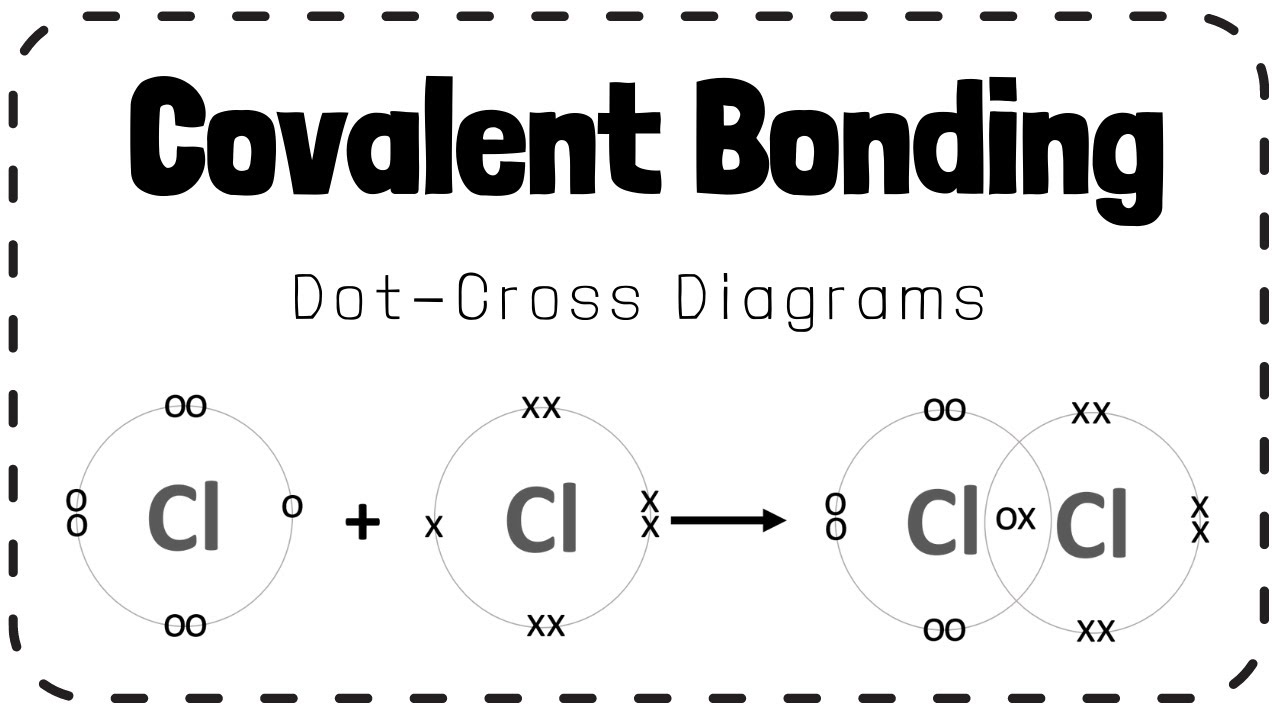
Covalent Bond . For full treatment, see chemical bonding: Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Find out how to work out the. — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. learn what a covalent bond is, how it is formed, and its properties and characteristics. — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. — learn how atoms share electrons to form covalent bonds and molecules. A brief treatment of covalent bonds follows.
from
— learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. learn what a covalent bond is, how it is formed, and its properties and characteristics. A brief treatment of covalent bonds follows. — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. Find out how to work out the. — learn how atoms share electrons to form covalent bonds and molecules. covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment.
Covalent Bond covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. A brief treatment of covalent bonds follows. Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. For full treatment, see chemical bonding: — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. — learn how atoms share electrons to form covalent bonds and molecules. Find out how to work out the. — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. learn what a covalent bond is, how it is formed, and its properties and characteristics. covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms.
From
Covalent Bond — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. — learn how atoms share electrons to form covalent bonds and molecules. covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Find out how to work out the. Explore. Covalent Bond.
From
Covalent Bond Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. — learn how atoms share electrons to form covalent bonds and molecules. — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. A brief treatment of covalent bonds follows. — learn what. Covalent Bond.
From www.slideserve.com
PPT Intro to Bonding Part 2 Covalent Compounds (Type 3 Binary Covalent Bond Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. — learn what a covalent bond. Covalent Bond.
From sansona.github.io
Attractive Forces and Bonds Covalent Bond learn what a covalent bond is, how it is formed, and its properties and characteristics. For full treatment, see chemical bonding: A brief treatment of covalent bonds follows. Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. — a covalent bond forms when the bonded atoms have a lower. Covalent Bond.
From library.fiveable.me
Strengths of Ionic and Covalent Bonds Intro to Chemistry Study Guide Covalent Bond Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. — learn how atoms share electrons to. Covalent Bond.
From
Covalent Bond For full treatment, see chemical bonding: learn what a covalent bond is, how it is formed, and its properties and characteristics. — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. Find out how to work out the. — learn how atoms share electrons to form covalent. Covalent Bond.
From sciencenotes.org
Covalent Compounds Examples and Properties Covalent Bond — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. A brief treatment of covalent bonds follows. For full treatment, see chemical bonding: learn what a covalent bond is, how it is formed, and. Covalent Bond.
From
Covalent Bond Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. learn what a covalent bond is, how it is formed, and its properties and characteristics. Find out how to work out the.. Covalent Bond.
From
Covalent Bond Find out how to work out the. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both. Covalent Bond.
From
Covalent Bond covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. —. Covalent Bond.
From animalia-life.club
Metallic Bond Examples List Covalent Bond — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. Find out. Covalent Bond.
From
Covalent Bond — learn how atoms share electrons to form covalent bonds and molecules. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. covalent bonds form when electrons are shared between atoms and are attracted. Covalent Bond.
From
Covalent Bond learn what a covalent bond is, how it is formed, and its properties and characteristics. A brief treatment of covalent bonds follows. For full treatment, see chemical bonding: covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Find out the properties and examples of covalent compounds and how they. Covalent Bond.
From
Covalent Bond learn what a covalent bond is, how it is formed, and its properties and characteristics. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. A brief treatment of covalent bonds follows.. Covalent Bond.
From
Covalent Bond For full treatment, see chemical bonding: Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. learn what a covalent bond is, how it is formed, and its properties and characteristics. — learn how atoms share electrons to form covalent bonds and molecules. — a covalent bond forms when the bonded atoms. Covalent Bond.
From
Covalent Bond Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. For full treatment, see chemical bonding: — learn how atoms share electrons to form covalent bonds and molecules. — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. covalent. Covalent Bond.
From www.mindomo.com
SNC1D0 Year Mind Map Covalent Bond Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. learn what a covalent bond is, how it is formed, and its properties and characteristics. Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. A brief treatment of covalent bonds follows.. Covalent Bond.
From
Covalent Bond — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. For full treatment, see chemical bonding: covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Explore the different types of covalent bonds based on the number of shared electrons,. Covalent Bond.
From
Covalent Bond — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. Find out the properties and. Covalent Bond.
From
Covalent Bond — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. learn what a covalent bond is, how it is formed, and its properties and characteristics. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. Explore how covalent bonds depend. Covalent Bond.
From
Covalent Bond A brief treatment of covalent bonds follows. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. learn what a covalent bond is, how it is formed, and its properties and characteristics. covalent bonds form. Covalent Bond.
From
Covalent Bond Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. learn what a covalent bond is, how it is formed, and its properties and characteristics. Explore how covalent bonds depend on. Covalent Bond.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Covalent Bond Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. — learn how atoms share electrons to form covalent bonds and molecules. For full treatment, see chemical bonding: — a covalent. Covalent Bond.
From mungfali.com
NH3 Covalent Bond Covalent Bond A brief treatment of covalent bonds follows. — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. learn what a covalent bond is, how it is formed, and its properties and. Covalent Bond.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples Covalent Bond Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. — learn how atoms share electrons to form covalent bonds and molecules. — a covalent bond forms when the bonded atoms have. Covalent Bond.
From
Covalent Bond — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. A brief treatment of covalent bonds. Covalent Bond.
From mavink.com
Polar Covalent Bond Model Covalent Bond Find out how to work out the. covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A brief treatment of covalent bonds follows. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. Find out the properties and examples of covalent compounds and how they. Covalent Bond.
From
Covalent Bond learn what a covalent bond is, how it is formed, and its properties and characteristics. — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms. — a covalent bond. Covalent Bond.
From
Covalent Bond Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Find out how to work out the. Explore the different types of covalent bonds based on the number of shared electrons, polarity, and coordination of atoms.. Covalent Bond.
From
Covalent Bond Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. Find out how to work out the. Find out the properties and examples of covalent compounds and how they differ from ionic and metallic bonds. learn what a covalent bond is, how it is formed, and its properties and characteristics. — learn the. Covalent Bond.
From
Covalent Bond — learn how atoms share electrons to form covalent bonds and molecules. — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. For full treatment, see chemical bonding: Explore the different types of covalent. Covalent Bond.
From
Covalent Bond — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. — learn the basic concepts and properties of covalent bonds, such as bond order, bond length, and bond energy. learn what a covalent bond is, how it is formed, and its properties and characteristics. Find out the properties and examples. Covalent Bond.
From
Covalent Bond For full treatment, see chemical bonding: — a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. — learn what a covalent bond is, how it forms, and what types. Covalent Bond.
From
Covalent Bond — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. For full treatment, see chemical bonding: covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. learn what a covalent bond is, how it is formed, and its properties and characteristics. . Covalent Bond.
From
Covalent Bond — learn what a covalent bond is, how it forms, and what types of covalent bonds exist. — learn how atoms share electrons to form covalent bonds and molecules. Explore how covalent bonds depend on the overlap of valence orbitals and the molecular environment. Find out how to work out the. Explore the different types of covalent bonds. Covalent Bond.