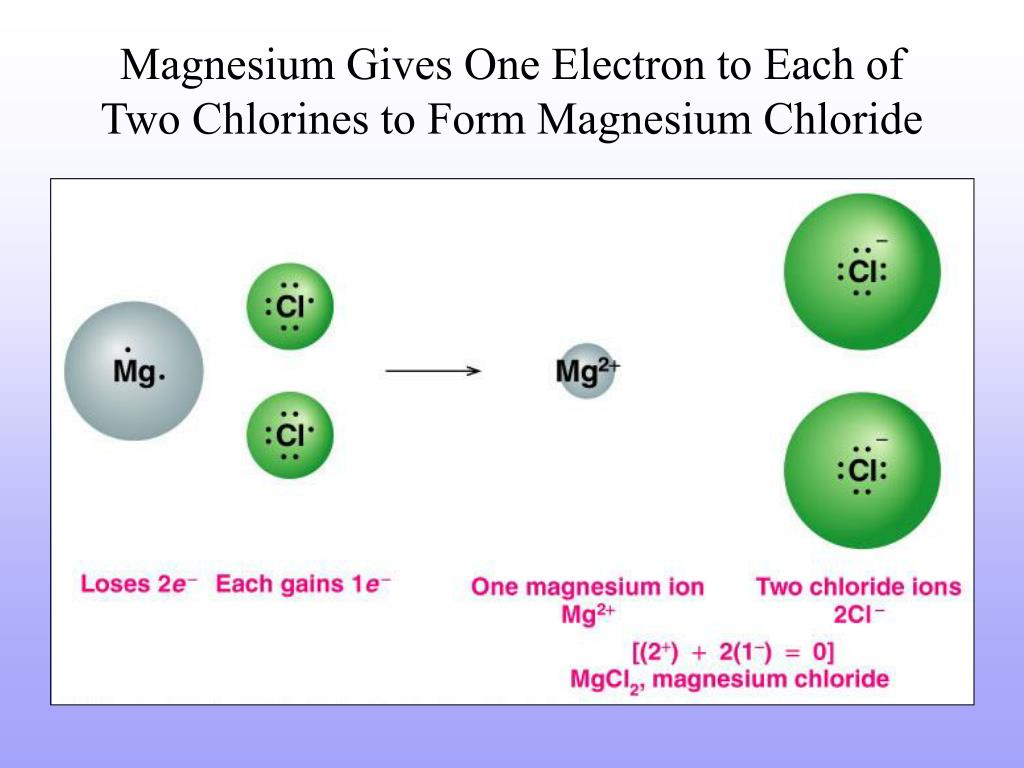
Magnesium Charge On Electrons . Magnesium ion(mg 2+) electron configuration. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. This electric charge generated on the ion is known. 93 rows the charge on an atom is related to its valence electrons or oxidation state. Magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. After the electron configuration, the last. The most common charges are based on maximum stability for the atom. It has an atomic weight of 24.305 and a. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).
from www.slideserve.com
The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². It has an atomic weight of 24.305 and a. This electric charge generated on the ion is known. The most common charges are based on maximum stability for the atom. 93 rows the charge on an atom is related to its valence electrons or oxidation state. Magnesium has two valence electrons in the 3s orbital. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Magnesium ion(mg 2+) electron configuration. After the electron configuration, the last.
PPT Valence Electrons PowerPoint Presentation, free download ID651377
Magnesium Charge On Electrons After the electron configuration, the last. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). After the electron configuration, the last. It has an atomic weight of 24.305 and a. Magnesium has two valence electrons in the 3s orbital. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Magnesium ion(mg 2+) electron configuration. The most common charges are based on maximum stability for the atom. The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. 93 rows the charge on an atom is related to its valence electrons or oxidation state. This electric charge generated on the ion is known. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Charge On Electrons After the electron configuration, the last. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The most common charges are based on maximum stability for the atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The ground state. Magnesium Charge On Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Magnesium Charge On Electrons The most common charges are based on maximum stability for the atom. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. After the electron configuration, the last. 93 rows. Magnesium Charge On Electrons.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Charge On Electrons It has an atomic weight of 24.305 and a. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. After the electron configuration, the last. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium ion(mg 2+) electron configuration. Magnesium is the 12th element in the periodic table and. Magnesium Charge On Electrons.
From www.dreamstime.com
Atom of Magnesium with Detailed Core and 12 Electrons on White with Magnesium Charge On Electrons The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². After the electron configuration, the last. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows the charge on an atom is. Magnesium Charge On Electrons.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Magnesium Charge On Electrons Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Magnesium ion(mg 2+) electron configuration. The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. The most common charges are based on maximum stability for the atom. 93 rows the charge on an atom. Magnesium Charge On Electrons.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Charge On Electrons 93 rows the charge on an atom is related to its valence electrons or oxidation state. This electric charge generated on the ion is known. After the electron configuration, the last. The most common charges are based on maximum stability for the atom. Magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of magnesium is. Magnesium Charge On Electrons.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Charge On Electrons Magnesium ion(mg 2+) electron configuration. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows the charge on an atom is related to its valence electrons or oxidation state. The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. After the electron. Magnesium Charge On Electrons.
From mavink.com
Draw The Orbital Diagram For Magnesium Magnesium Charge On Electrons The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². It has an atomic weight of 24.305 and a. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. When. Magnesium Charge On Electrons.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Magnesium Charge On Electrons The most common charges are based on maximum stability for the atom. Magnesium ion(mg 2+) electron configuration. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². After the electron configuration, the last. Magnesium has two valence electrons in the 3s orbital. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion. Magnesium Charge On Electrons.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Charge On Electrons 93 rows the charge on an atom is related to its valence electrons or oxidation state. Magnesium ion(mg 2+) electron configuration. After the electron configuration, the last. This electric charge generated on the ion is known. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. The electron configuration of. Magnesium Charge On Electrons.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Charge On Electrons Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. It has an atomic weight of 24.305 and a. After the electron configuration, the last. The most common charges are based on maximum stability for the atom. This electric charge generated on the ion is known. Magnesium has two valence. Magnesium Charge On Electrons.
From www.pinterest.se
GensonScience Magnesium Atom model project, Atom model, Atoms and Magnesium Charge On Electrons When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. This electric charge generated on the ion is known. Magnesium has two valence electrons in the 3s orbital. It has an atomic weight of. Magnesium Charge On Electrons.
From www.bigstockphoto.com
3d Render Atom Structure Magnesium Image & Photo Bigstock Magnesium Charge On Electrons Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. This electric charge generated on the ion is known. The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. It has an atomic weight of 24.305 and a. Magnesium has two valence electrons in. Magnesium Charge On Electrons.
From www.toppr.com
Write the electron dot structure of magnesium and oxygen. Magnesium Charge On Electrons This electric charge generated on the ion is known. The most common charges are based on maximum stability for the atom. Magnesium ion(mg 2+) electron configuration. Magnesium has two valence electrons in the 3s orbital. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. Magnesium is the 12th element. Magnesium Charge On Electrons.
From www.dreamstime.com
Electron of the Element Magnesium Stock Vector Illustration of Magnesium Charge On Electrons The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. This electric charge generated on the ion is known. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron affinity the energy released. Magnesium Charge On Electrons.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Magnesium Charge On Electrons Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). After the electron configuration, the last. The most common charges are based on maximum stability for the atom. Electron affinity. Magnesium Charge On Electrons.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition, electron Magnesium Charge On Electrons The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. This electric charge generated on the ion is known. 93 rows the charge on an atom is related to its valence electrons or oxidation state. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². After the electron configuration, the last. Magnesium ion(mg 2+). Magnesium Charge On Electrons.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Magnesium Charge On Electrons After the electron configuration, the last. Magnesium has two valence electrons in the 3s orbital. This electric charge generated on the ion is known. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Magnesium ion(mg 2+) electron configuration. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The. Magnesium Charge On Electrons.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Charge On Electrons This electric charge generated on the ion is known. Magnesium ion(mg 2+) electron configuration. It has an atomic weight of 24.305 and a. The most common charges are based on maximum stability for the atom. After the electron configuration, the last. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital.. Magnesium Charge On Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Charge On Electrons Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. This electric charge generated on the ion is known. The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is. Magnesium Charge On Electrons.
From www.slideserve.com
PPT Valence Electrons PowerPoint Presentation, free download ID651377 Magnesium Charge On Electrons Magnesium has two valence electrons in the 3s orbital. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows the charge on an atom is related to its valence electrons or oxidation state. The most common charges are based on maximum stability for the atom. Electron affinity the. Magnesium Charge On Electrons.
From www.alamyimages.fr
Le magnésium (Mg). Schéma de la composition nucléaire et configuration Magnesium Charge On Electrons The most common charges are based on maximum stability for the atom. This electric charge generated on the ion is known. Magnesium has two valence electrons in the 3s orbital. 93 rows the charge on an atom is related to its valence electrons or oxidation state. Electron affinity the energy released when an electron is added to the neutral atom. Magnesium Charge On Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Charge On Electrons It has an atomic weight of 24.305 and a. After the electron configuration, the last. The most common charges are based on maximum stability for the atom. 93 rows the charge on an atom is related to its valence electrons or oxidation state. Magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of magnesium is. Magnesium Charge On Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Charge On Electrons When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). It has an atomic weight of 24.305 and a. Magnesium ion(mg 2+) electron configuration. The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. This electric charge generated on the ion is known. Magnesium. Magnesium Charge On Electrons.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Charge On Electrons The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. This electric charge generated. Magnesium Charge On Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Charge On Electrons Magnesium has two valence electrons in the 3s orbital. It has an atomic weight of 24.305 and a. This electric charge generated on the ion is known. The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. The most common charges are based on maximum stability for the atom. After the electron configuration, the. Magnesium Charge On Electrons.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Charge On Electrons Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. This electric charge generated on the ion is known. Magnesium has two valence electrons in the 3s orbital. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. The ground. Magnesium Charge On Electrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Charge On Electrons The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. After the electron configuration, the last. Magnesium has two valence electrons in the 3s orbital. The most common charges are based on maximum stability. Magnesium Charge On Electrons.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Charge On Electrons The most common charges are based on maximum stability for the atom. It has an atomic weight of 24.305 and a. The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron affinity the energy released when an electron is added to the neutral. Magnesium Charge On Electrons.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Magnesium Charge On Electrons When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). It has an atomic weight of 24.305 and a. The most common charges are based on maximum stability for the atom. This electric charge generated on the ion is known. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².. Magnesium Charge On Electrons.
From commons.wikimedia.org
FileElectron shell 012 Magnesium.svg Wikimedia Commons Magnesium Charge On Electrons Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of magnesium is 1s 2 2s. Magnesium Charge On Electrons.
From www.alamyimages.fr
Le magnésium (Mg). Schéma de la composition nucléaire et configuration Magnesium Charge On Electrons When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Magnesium has two valence electrons in the 3s orbital. It has an atomic weight of 24.305 and a. The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. The electron configuration of magnesium is. Magnesium Charge On Electrons.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Charge On Electrons Magnesium ion(mg 2+) electron configuration. Electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s. Magnesium Charge On Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Charge On Electrons The most common charges are based on maximum stability for the atom. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². It has an atomic weight of 24.305 and a. This electric charge generated on the ion is known. After the electron configuration, the last. 93 rows the charge on an atom is related to its valence electrons or. Magnesium Charge On Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Charge On Electrons After the electron configuration, the last. This electric charge generated on the ion is known. Magnesium has two valence electrons in the 3s orbital. Magnesium ion(mg 2+) electron configuration. 93 rows the charge on an atom is related to its valence electrons or oxidation state. The most common charges are based on maximum stability for the atom. The ground state. Magnesium Charge On Electrons.