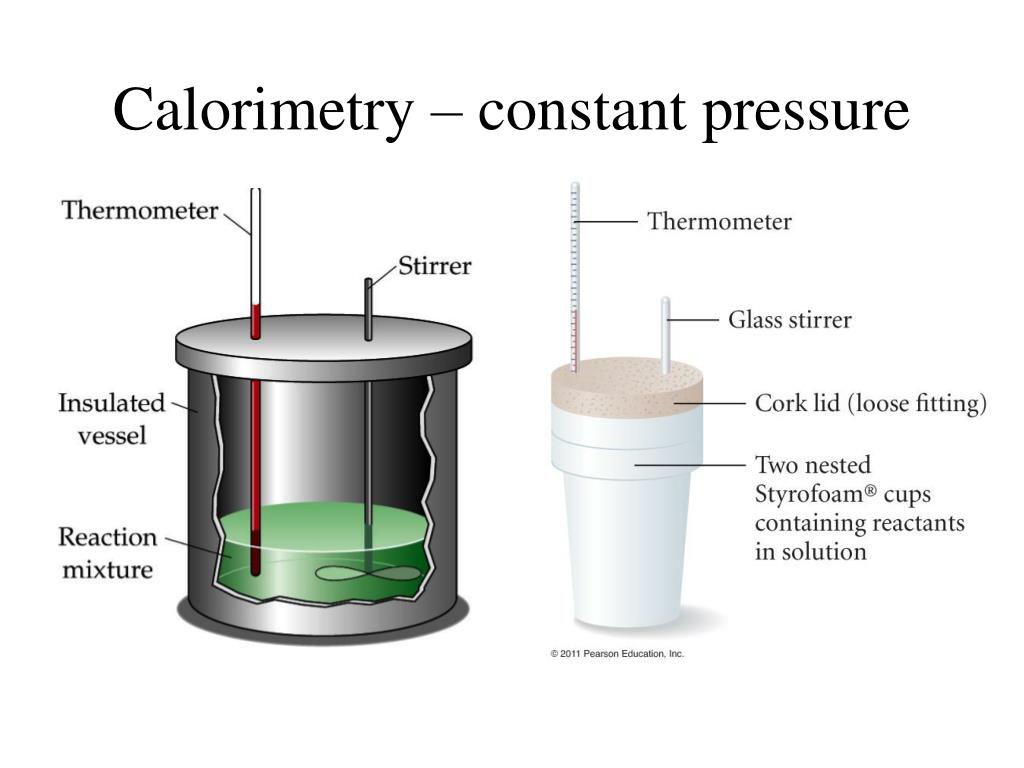
Calorimeter Def . Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. For example, when an exothermic. The process of measuring this heat is called calorimetry. Calorimeters have been designed in great variety. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from www.slideserve.com
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. The process of measuring this heat is called calorimetry. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeters have been designed in great variety.
PPT Thermochemistry PowerPoint Presentation, free download ID2692866
Calorimeter Def Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimeters have been designed in great variety. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Calorimeter Def Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeters have been designed in great variety. For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called calorimetry. The meaning of. Calorimeter Def.
From courses.lumenlearning.com
5.2 Calorimetry Chemistry Calorimeter Def For example, when an exothermic. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of. Calorimeter Def.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Calorimeter Def A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The meaning of calorimeter is. Calorimeter Def.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Calorimeter Def Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters have been designed in great variety. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. A calorimeter is a device used to measure the heat flow. Calorimeter Def.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Def For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeter,. Calorimeter Def.
From studylib.net
Calorimetry Calorimeter Def Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. Calorimeters have been designed in great variety. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. Calorimeter Def.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Def Calorimeters have been designed in great variety. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. A calorimeter is a device used to measure the amount of. Calorimeter Def.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Def The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using. Calorimeter Def.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Def Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or. Calorimeter Def.
From www.slideserve.com
PPT a “ Calorimeter ” PowerPoint Presentation, free download ID7050684 Calorimeter Def Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters have been designed in great variety. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes. Calorimeter Def.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Def Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeters have been designed in great variety. Calorimetry is the process of measuring the amount. Calorimeter Def.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Calorimeter Def A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimeter, device for measuring. Calorimeter Def.
From courses.lumenlearning.com
9.2 Calorimetry General College Chemistry I Calorimeter Def The process of measuring this heat is called calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the heat flow of. Calorimeter Def.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Def A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeters have been designed in. Calorimeter Def.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Calorimeter Def Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeters have been designed in great variety. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called calorimetry. Calorimetry describes a set of techniques employed to. Calorimeter Def.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter Def A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called calorimetry. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. Calorimeter Def.
From glossary.periodni.com
Download calorimeter.png image from Calorimeter Def Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters have. Calorimeter Def.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Def A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called calorimetry. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a. Calorimeter Def.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Def Calorimeters have been designed in great variety. For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The meaning of calorimeter is an apparatus for. Calorimeter Def.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Calorimeter Def Calorimeters have been designed in great variety. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimeter Def.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimeter Def A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeters. Calorimeter Def.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimeter Def Calorimeters have been designed in great variety. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimeter Def.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Def The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters have been designed. Calorimeter Def.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Def A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry describes a. Calorimeter Def.
From saylordotorg.github.io
Calorimetry Calorimeter Def Calorimeters have been designed in great variety. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The meaning of calorimeter is an apparatus for measuring quantities of absorbed. Calorimeter Def.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Def The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved. Calorimeter Def.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeter Def For example, when an exothermic. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. The process of measuring this heat is called calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used. Calorimeter Def.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Def A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used. Calorimeter Def.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimeter Def Calorimeters have been designed in great variety. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. For example, when an exothermic. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. The meaning of calorimeter is an apparatus for measuring quantities of absorbed. Calorimeter Def.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Def Calorimeters have been designed in great variety. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for. Calorimeter Def.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Def The process of measuring this heat is called calorimetry. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeters have been designed in great variety. Calorimeter, device for measuring. Calorimeter Def.
From gamesmartz.com
Calorimeter Definition Easy to Understand Calorimeter Def The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an. Calorimeter Def.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture Calorimeter Def The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when. Calorimeter Def.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Def Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimeter Def.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Calorimeter Def Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The meaning of calorimeter is an apparatus for measuring quantities of absorbed or emitted heat or for determining specific heats. Calorimeters. Calorimeter Def.