Usp Incubator Validation . the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. Media filled isolates are identified by. several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the.
from www.slideshare.net
the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. Media filled isolates are identified by. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on.
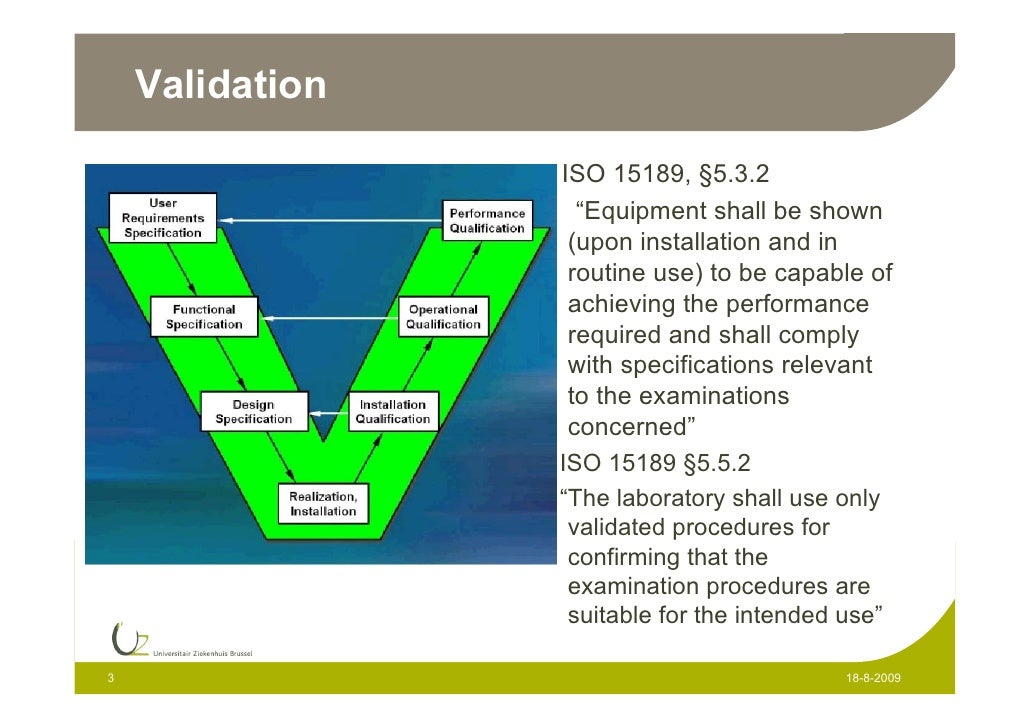
Ronny Janssens Validation G 185 Incubator
Usp Incubator Validation the validation process is generally made employing product inoculated with appropriate (bis) such as spore. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. Media filled isolates are identified by. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days.
From www.slideshare.net
Analytical Method Validation as per ICH vs USP Usp Incubator Validation the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the authors believe this review makes a strong case that colony counters such as. Usp Incubator Validation.
From www.qimedical.com
QuickTest™ System QT1000 Aseptic Process Validation Testing Kit Usp Incubator Validation the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the authors believe this review makes a strong case that colony counters such as. Usp Incubator Validation.
From www.slideshare.net
Ronny Janssens Validation G 185 Incubator Usp Incubator Validation the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. several criteria should be considered when. Usp Incubator Validation.
From pharmadekho.com
sop for validation of incubators Pharma Dekho Usp Incubator Validation several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. Media filled isolates are identified by. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. the incubation period of a media fill should be no less than 14 days and the containers should. Usp Incubator Validation.
From pharmadekho.com
sop for validation of incubators Pharma Dekho Usp Incubator Validation the validation process is generally made employing product inoculated with appropriate (bis) such as spore. Media filled isolates are identified by. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the incubation period of a media fill should be no less than 14 days and the containers. Usp Incubator Validation.
From www.kayeinstruments.com
IRTD400 Usp Incubator Validation Media filled isolates are identified by. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the validation process is generally made employing product inoculated with appropriate (bis) such as. Usp Incubator Validation.
From globallabsupplyca.wordpress.com
What Exactly is an Incubator? The Complete Guide To Understanding Usp Incubator Validation the validation process is generally made employing product inoculated with appropriate (bis) such as spore. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated. Usp Incubator Validation.
From www.slideshare.net
Analytical Method Validation as per ICH vs USP Usp Incubator Validation the validation process is generally made employing product inoculated with appropriate (bis) such as spore. Media filled isolates are identified by. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. this validation follows the procedure described for validation test under. Usp Incubator Validation.
From www.ellab.com
Incubator Validation Pharmaceutical Industry Usp Incubator Validation the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the validation process is generally made employing product inoculated with appropriate (bis) such. Usp Incubator Validation.
From www.slideserve.com
PPT Why LowTemperature? PowerPoint Presentation, free download ID Usp Incubator Validation the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. this validation follows the procedure described for validation test under sterility tests 71, with. Usp Incubator Validation.
From www.thermalcompliance.com
Validation 28 Temperature Mapping of C02 Incubators Therm Usp Incubator Validation this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. the incubation period of a media fill should be no less than. Usp Incubator Validation.
From www.kenelec.com.au
Monitoring, Validation and Mapping in Incubators and Test Chambers Usp Incubator Validation this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or. Usp Incubator Validation.
From www.indiamart.com
Bod Incubator Validation Service at best price in Valsad ID 25701141312 Usp Incubator Validation several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. Media filled isolates are identified by. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the validation process is generally made employing product inoculated with appropriate (bis) such as. Usp Incubator Validation.
From www.esco-medical.com
News Advantages of Using MIRI® GA in Validation of IVF Incubators Usp Incubator Validation several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. the validation process is generally made employing product inoculated with appropriate (bis) such as. Usp Incubator Validation.
From www.slideserve.com
PPT Why LowTemperature? PowerPoint Presentation, free download ID Usp Incubator Validation this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. the incubation period of a media fill should be no less than. Usp Incubator Validation.
From microbe-investigations.com
USP 61 Testing for Microbial Enumeration Microbe Investigations Usp Incubator Validation the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. Media filled isolates are identified by. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. this validation follows the procedure described for validation test under sterility. Usp Incubator Validation.
From www.researchgate.net
Methods of validation for the USP cases Download Table Usp Incubator Validation the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. Media filled isolates are identified by. the validation process is generally made employing. Usp Incubator Validation.
From pharmadekho.com
sop for validation of incubators Pharma Dekho Usp Incubator Validation Media filled isolates are identified by. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. the incubation period of a media fill should be no less than 14 days and the containers. Usp Incubator Validation.
From www.igenels.com
CO2 Incubator IGC Series iGene Labserve Usp Incubator Validation the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. Media filled isolates are identified by. several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the incubation period of a media fill. Usp Incubator Validation.
From www.slideshare.net
Method Validation ICH /USP Validation, Linearity and Repeatability Usp Incubator Validation the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. the incubation period of a media fill should be no less than 14 days and the. Usp Incubator Validation.
From labmonk.com
USP Mycoplasma Testing An Overview Labmonk Usp Incubator Validation the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the validation process is generally made employing product inoculated with appropriate (bis) such. Usp Incubator Validation.
From www.youtube.com
Biological incubator How to read biological indicator Gke Sterl Usp Incubator Validation Media filled isolates are identified by. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. several criteria should be considered when selecting a method to. Usp Incubator Validation.
From anatune.co.uk
Blog Alternative Procedure for USP Using SIFTMS Usp Incubator Validation the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. this validation follows the procedure described for validation test under sterility tests 71, with the exception. Usp Incubator Validation.
From www.scribd.com
Validation of Incubators PDF Thermometer Chemistry Usp Incubator Validation several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. the incubation period of a media fill should be no less than 14. Usp Incubator Validation.
From www.indiamart.com
Incubator Temperature Mapping & Validation Service at Rs 5500/hour in Usp Incubator Validation the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. several criteria should be considered when. Usp Incubator Validation.
From www.slideshare.net
Ronny Janssens Validation G 185 Incubator Usp Incubator Validation the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. Media filled isolates are identified by. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. the incubation period of a media fill should be no. Usp Incubator Validation.
From www.pharmaqualification.com
Incubator Validation Protocol PDF included Usp Incubator Validation several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3 days. the validation process is generally made employing product inoculated with appropriate (bis) such as. Usp Incubator Validation.
From www.slideshare.net
Method Validation ICH /USP Validation, Linearity and Repeatability Usp Incubator Validation Media filled isolates are identified by. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. several criteria should be considered when selecting a method to. Usp Incubator Validation.
From www.scribd.com
Incubators Validation PDF Usp Incubator Validation the validation process is generally made employing product inoculated with appropriate (bis) such as spore. several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the incubation period of a media fill should be no less than 14 days and the containers should be examined every 2 or 3. Usp Incubator Validation.
From www.biomedqc.com
BioMed QC USP 797 Compliance What is VeriGrow? Usp Incubator Validation the validation process is generally made employing product inoculated with appropriate (bis) such as spore. Media filled isolates are identified by. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. this validation follows the procedure described for validation test under. Usp Incubator Validation.
From www.newtonsmedicalsupplies.co.uk
Validation Kit for Mini Pro Incubator Newtons Medical Supplies Usp Incubator Validation Media filled isolates are identified by. this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. the incubation period of a media. Usp Incubator Validation.
From www.powershow.com
PPT Laboratory incubators PowerPoint presentation free to download Usp Incubator Validation the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. Media filled isolates are identified by. several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the validation process is generally made employing. Usp Incubator Validation.
From www.scribd.com
Installation / Operational Qualification Protocol Insert Incubator Name Usp Incubator Validation this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated. Usp Incubator Validation.
From www.phchd.com
Laboratory Equipment Validation PHCbi Usp Incubator Validation this validation follows the procedure described for validation test under sterility tests 71, with the exception of plating on. the authors believe this review makes a strong case that colony counters such as the growth direct ® system qualify as an automated system for the. the incubation period of a media fill should be no less than. Usp Incubator Validation.
From nitrosamines.usp.org
Discussion on Cleaning Validation Requirements for Nitrosamines New Usp Incubator Validation several criteria should be considered when selecting a method to monitor the microbial content of a pharmaceutical water. the validation process is generally made employing product inoculated with appropriate (bis) such as spore. Media filled isolates are identified by. the incubation period of a media fill should be no less than 14 days and the containers should. Usp Incubator Validation.