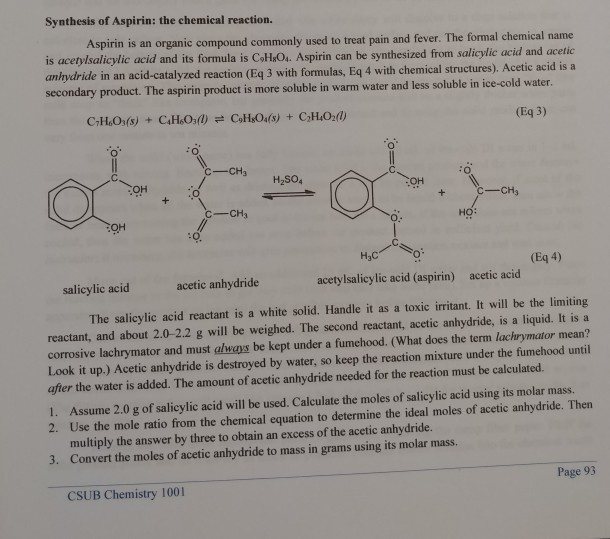
Aspirin Chemical Equation Reaction . the reaction is catalyzed by concentrated phosphoric acid. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. conduct a chemical reaction to produce aspirin. the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. Aspirin is prepared by chemical synthesis from. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. Excess acetic hydride may be used in this reaction as.
from www.chegg.com
the reaction is catalyzed by concentrated phosphoric acid. Aspirin is prepared by chemical synthesis from. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin. conduct a chemical reaction to produce aspirin. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. Excess acetic hydride may be used in this reaction as.
Solved Synthesis of Aspirin the chemical reaction. Aspirin
Aspirin Chemical Equation Reaction synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. Excess acetic hydride may be used in this reaction as. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. the reaction is catalyzed by concentrated phosphoric acid. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. conduct a chemical reaction to produce aspirin. chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. Aspirin is prepared by chemical synthesis from. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin.
From www.tessshebaylo.com
Chemical Equation Representing Synthesis Of Aspirin From Acetyl Chloride Tessshebaylo Aspirin Chemical Equation Reaction conduct a chemical reaction to produce aspirin. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. the reaction is catalyzed by concentrated phosphoric acid. Excess acetic hydride may be used in this reaction as. it uses a reaction of esterification catalyzed acid (h. Aspirin Chemical Equation Reaction.
From www.pinterest.com
Images For > Aspirin Molecular Structure Molecular structure, Molecular, Chemistry Aspirin Chemical Equation Reaction Excess acetic hydride may be used in this reaction as. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. conduct a chemical reaction to produce aspirin. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product. Aspirin Chemical Equation Reaction.
From www.hotelsrate.org
Chemical Equation Synthesis Of Aspirin From Acetyl Chloride Diy Projects Aspirin Chemical Equation Reaction Excess acetic hydride may be used in this reaction as. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin. the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate. Aspirin Chemical Equation Reaction.
From chemistrylives.blogspot.com
Practical2 Reaction Mechanism of Aspirin (steps for the preparation through chemical approach) Aspirin Chemical Equation Reaction it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. Aspirin is prepared by chemical synthesis from. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. The molecular formula. Aspirin Chemical Equation Reaction.
From www.chegg.com
Solved Synthesis of Aspirin the chemical reaction. Aspirin Aspirin Chemical Equation Reaction Excess acetic hydride may be used in this reaction as. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. chemical formula = c9h8o4 or ch3cooc6h4cooh or. Aspirin Chemical Equation Reaction.
From www.tessshebaylo.com
Balanced Chemical Equation For The Synthesis Of Aspirin Using Salicylic Acid And Acetic Aspirin Chemical Equation Reaction The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h. Aspirin Chemical Equation Reaction.
From www.tessshebaylo.com
Chemical Equation Representing Synthesis Of Aspirin From Acetyl Chloride Tessshebaylo Aspirin Chemical Equation Reaction Aspirin is prepared by chemical synthesis from. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. the reaction is catalyzed by concentrated phosphoric acid. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where. Aspirin Chemical Equation Reaction.
From www.studocu.com
Lab 4 Synthesis of Aspirin Lab Partner_____________________________ Synthesis of Aspirin Week Aspirin Chemical Equation Reaction conduct a chemical reaction to produce aspirin. Excess acetic hydride may be used in this reaction as. chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. the reaction is catalyzed by. Aspirin Chemical Equation Reaction.
From www.numerade.com
SOLVED Write balanced reaction equations for the reactions involved (a) when aspirin dissolves Aspirin Chemical Equation Reaction the reaction is catalyzed by concentrated phosphoric acid. Excess acetic hydride may be used in this reaction as. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. conduct a chemical reaction to produce aspirin. chemical formula = c9h8o4 or. Aspirin Chemical Equation Reaction.
From byjus.com
Aspirin Formula Structural and Chemical Formula of Aspirin Aspirin Chemical Equation Reaction chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. Excess acetic hydride may be used in this reaction as. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your. Aspirin Chemical Equation Reaction.
From www.saubhaya.com
Chemical Makeup Of Aspirin Saubhaya Makeup Aspirin Chemical Equation Reaction The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to. Aspirin Chemical Equation Reaction.
From www.freepik.com
Premium Vector Aspirin chemistry chemical formula structure vector Aspirin Chemical Equation Reaction chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. Aspirin is prepared by chemical synthesis from. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. the reaction is catalyzed by concentrated phosphoric acid. synthesize acetylsalicylic acid (aspirin) by carrying out a simple. Aspirin Chemical Equation Reaction.
From www.chegg.com
Solved 2. Learning how fast the hydrolysis of aspirin occurs Aspirin Chemical Equation Reaction synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. the reaction is catalyzed by concentrated phosphoric acid. chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where. Aspirin Chemical Equation Reaction.
From pixelrz.com
Aspirine Synthese Aspirin Chemical Equation Reaction Excess acetic hydride may be used in this reaction as. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. Aspirin is prepared by chemical synthesis from. conduct a chemical reaction to produce aspirin. The molecular formula for acetylsalicylic acid is c. Aspirin Chemical Equation Reaction.
From www.numerade.com
SOLVED 1. Write the balanced chemical formula of the reaction of aspirin and NaOH. 2. Write the Aspirin Chemical Equation Reaction the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin. conduct a chemical reaction to produce aspirin. Excess acetic hydride may be used. Aspirin Chemical Equation Reaction.
From www.reddit.com
Do these reactions make sense? Asked to show chemical equations for when aspirin reacts with Aspirin Chemical Equation Reaction Excess acetic hydride may be used in this reaction as. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives.. Aspirin Chemical Equation Reaction.
From www.vrogue.co
Illustrated Glossary Of Organic Chemistry Aspirin Ace vrogue.co Aspirin Chemical Equation Reaction the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin. conduct a chemical reaction to produce aspirin. Excess acetic hydride may be used in this reaction as. Aspirin is prepared by chemical. Aspirin Chemical Equation Reaction.
From www.numerade.com
SOLVED ..1 0('n1.'41 . /' ^!.130 L ) ^ '" Calculations Determine the yield of the aspirin Aspirin Chemical Equation Reaction the reaction is catalyzed by concentrated phosphoric acid. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin. the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and. Aspirin Chemical Equation Reaction.
From study.com
Hydrolysis of Aspirin Overview, Reactions & Mechanism Lesson Aspirin Chemical Equation Reaction Aspirin is prepared by chemical synthesis from. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. the reaction is catalyzed by concentrated phosphoric acid. Excess acetic hydride may be used in this reaction as. it uses a reaction of esterification catalyzed acid. Aspirin Chemical Equation Reaction.
From www.dreamstime.com
Aspirin molecule stock vector. Illustration of chemistry 29406923 Aspirin Chemical Equation Reaction The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. Excess acetic hydride may be used in this reaction as. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic. Aspirin Chemical Equation Reaction.
From www.coursehero.com
[Solved] please draw an organic chemistry reaction of synthesis of aspirin... Course Hero Aspirin Chemical Equation Reaction the reaction is catalyzed by concentrated phosphoric acid. conduct a chemical reaction to produce aspirin. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. Aspirin is prepared by chemical synthesis from. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4. Aspirin Chemical Equation Reaction.
From momentumclubs.org
😂 Aspirin synthesis reaction. What Is the Chemical Equation for the Synthesis of Aspirin?. 2019 Aspirin Chemical Equation Reaction conduct a chemical reaction to produce aspirin. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin. Aspirin is prepared by chemical. Aspirin Chemical Equation Reaction.
From www.chegg.com
Solved Give the reaction mechanism for the synthesis of Aspirin Chemical Equation Reaction conduct a chemical reaction to produce aspirin. the reaction is catalyzed by concentrated phosphoric acid. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin.. Aspirin Chemical Equation Reaction.
From barmettler38394.blogspot.com
Synthesis Of Aspirin Without Acetic Anhydride Aspirin Chemical Equation Reaction synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. Aspirin is prepared by chemical synthesis from. chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc. Aspirin Chemical Equation Reaction.
From ibalchemy.com
D.2 Aspirin and penicillin IB Alchemy Aspirin Chemical Equation Reaction chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. the reaction is catalyzed by concentrated phosphoric acid. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic. Aspirin Chemical Equation Reaction.
From www.dreamstime.com
Aspirin Stock Vector Image 42307258 Aspirin Chemical Equation Reaction The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. Excess acetic hydride may be used in this reaction as. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin. it uses a reaction. Aspirin Chemical Equation Reaction.
From www.numerade.com
SOLVED 6 In the synthesis of aspirin we react salicylic acid with acetic anhydride. The Aspirin Chemical Equation Reaction Aspirin is prepared by chemical synthesis from. Excess acetic hydride may be used in this reaction as. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from. Aspirin Chemical Equation Reaction.
From www.coursehero.com
[Solved] Please write down the chemical equations of aspirin synthesis and... Course Hero Aspirin Chemical Equation Reaction The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. conduct a chemical reaction to produce aspirin. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. the neutralization reaction. Aspirin Chemical Equation Reaction.
From www.youtube.com
Solubility of Aspirin (Explained) YouTube Aspirin Chemical Equation Reaction the reaction is catalyzed by concentrated phosphoric acid. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. Aspirin is prepared. Aspirin Chemical Equation Reaction.
From www.alamy.com
Aspirin, formula and molecular structure. Acetylsalicylic acid, ASA. Medication used to reduce Aspirin Chemical Equation Reaction Excess acetic hydride may be used in this reaction as. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. it. Aspirin Chemical Equation Reaction.
From www.coursehero.com
[Solved] POSTLAB 1. The reaction for the synthesis of aspirin is given... Course Hero Aspirin Chemical Equation Reaction conduct a chemical reaction to produce aspirin. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. Excess acetic hydride may be used in this reaction as. synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product. Aspirin Chemical Equation Reaction.
From www.slideserve.com
PPT Aspirin Synthesis PowerPoint Presentation, free download ID1221482 Aspirin Chemical Equation Reaction the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin. chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. Aspirin is prepared by chemical synthesis from. conduct a chemical reaction to produce. Aspirin Chemical Equation Reaction.
From www.slideserve.com
PPT CH 104 SYNTHESIS OF ASPIRIN AND OIL OF WINTERGREEN PowerPoint Presentation ID4866738 Aspirin Chemical Equation Reaction chemical formula = c9h8o4 or ch3cooc6h4cooh or hc9h7o4. conduct a chemical reaction to produce aspirin. the reaction is catalyzed by concentrated phosphoric acid. the neutralization reaction can be used to determine the amount of aspirin (acetylsalicylic acid) present in. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula. Aspirin Chemical Equation Reaction.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation ID6186176 Aspirin Chemical Equation Reaction synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify. conduct a chemical reaction to produce aspirin. the reaction is catalyzed by concentrated phosphoric acid. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the. Aspirin Chemical Equation Reaction.
From www.numerade.com
SOLVED Practice Exercise 3.34 Synthesis of Aspirin In the synthesis of aspirin, we react Aspirin Chemical Equation Reaction Aspirin is prepared by chemical synthesis from. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives. . Aspirin Chemical Equation Reaction.