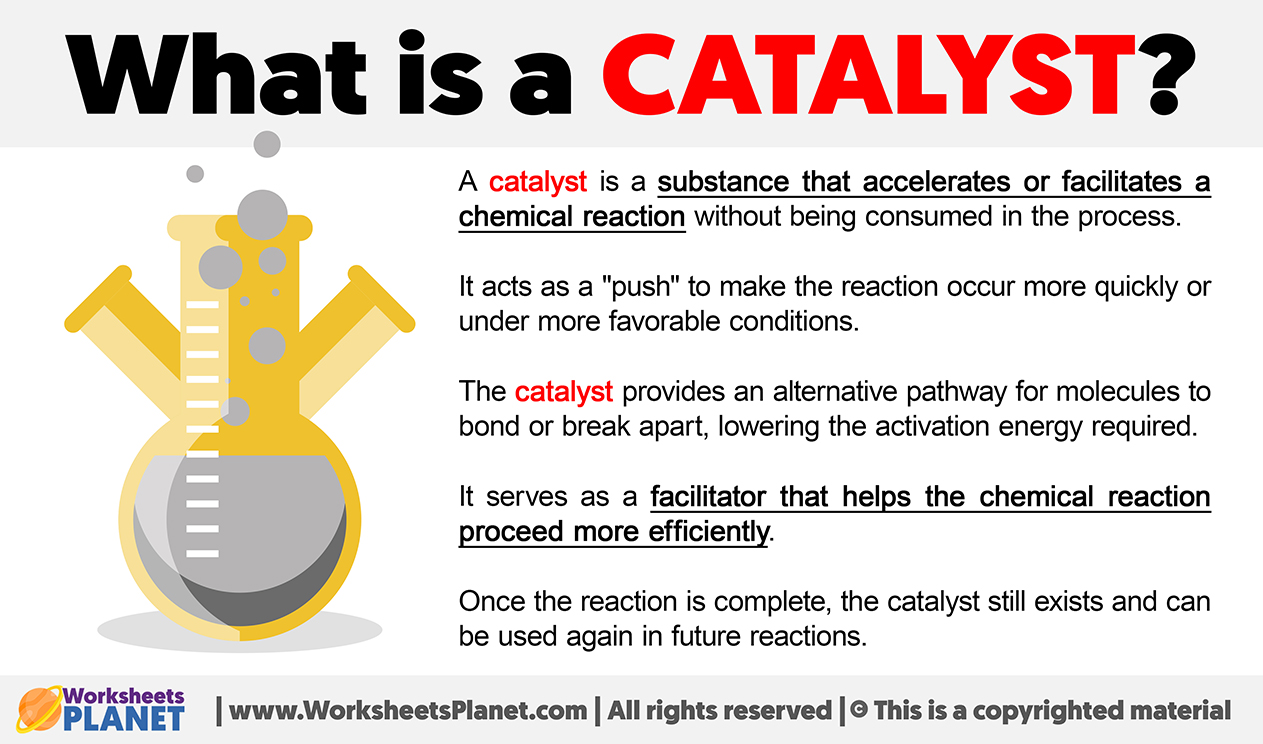
Catalyst Definition Gcse . a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. This pathway has a lower activation energy,. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; This means that the mass of a. Increases the rate of a reaction. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Hence a catalyst can be recovered chemically. Is not chemically changed or used up at the end of the. They do this by changing the reaction pathway. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel) study guide. A catalyst is often used to make a reaction go faster. catalysts increase the rate of chemical reactions by lowering the activation energy. a catalyst is a substance that will change the rate of a reaction.
from www.worksheetsplanet.com
catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. This means that the mass of a. A catalyst is often used to make a reaction go faster. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Is not chemically changed or used up at the end of the. learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel) study guide. This pathway has a lower activation energy,. catalysts increase the rate of chemical reactions by lowering the activation energy. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. a catalyst is a substance that will change the rate of a reaction.
What is a Catalyst Definition of Catalyst
Catalyst Definition Gcse a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. Hence a catalyst can be recovered chemically. They do this by changing the reaction pathway. catalysts increase the rate of chemical reactions by lowering the activation energy. Is not chemically changed or used up at the end of the. a catalyst is a substance that will change the rate of a reaction. a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. A catalyst is often used to make a reaction go faster. This pathway has a lower activation energy,. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Increases the rate of a reaction. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. This means that the mass of a. learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel) study guide.
From dxoweljvq.blob.core.windows.net
What Is A Catalyst Gcse Chemistry at Daniel Pham blog Catalyst Definition Gcse This means that the mass of a. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Is not chemically changed or used up at the end of the. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. Hence a catalyst can. Catalyst Definition Gcse.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Definition Gcse Increases the rate of a reaction. A catalyst is often used to make a reaction go faster. This pathway has a lower activation energy,. They do this by changing the reaction pathway. a catalyst is a substance that will change the rate of a reaction. a catalyst is a substance that increases the rate of a chemical reaction,. Catalyst Definition Gcse.
From exobfetmk.blob.core.windows.net
Examples Of Catalysts Gcse Chemistry at Colin Aleman blog Catalyst Definition Gcse This means that the mass of a. a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. Increases the rate of a reaction. learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel) study guide. This pathway has a lower activation energy,. Is. Catalyst Definition Gcse.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Presentation ID1988156 Catalyst Definition Gcse a catalyst is a substance that will change the rate of a reaction. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Increases the rate of a reaction. They do this by changing the reaction pathway. This means that the mass of a. catalysts increase the rate. Catalyst Definition Gcse.
From exoddixpp.blob.core.windows.net
Define Catalyst Science Term at Michael Moorehead blog Catalyst Definition Gcse Increases the rate of a reaction. Hence a catalyst can be recovered chemically. This means that the mass of a. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. catalysts increase the rate of chemical reactions by lowering the activation energy. A catalyst is often used to make. Catalyst Definition Gcse.
From www.pinterest.ca
Catalyst and Mechanism of Enzyme Catalysis Enzymes, Chemistry, Active site Catalyst Definition Gcse Increases the rate of a reaction. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Is not chemically changed or used up at the end of the. learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel) study guide. A catalyst is often. Catalyst Definition Gcse.
From www.youtube.com
Definition of Catalyst Chemical Chemistry Class 12 YouTube Catalyst Definition Gcse This pathway has a lower activation energy,. catalysts increase the rate of chemical reactions by lowering the activation energy. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Hence a catalyst can be recovered chemically. a catalyst is a substance that increases the rate of a chemical reaction,. Catalyst Definition Gcse.
From www.tes.com
Catalyst GCSE Chemistry Lesson PowerPoint (EDEXCEL) Teaching Resources Catalyst Definition Gcse This means that the mass of a. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; catalysts increase the rate of chemical reactions by lowering the activation energy. Increases the rate of a reaction. Hence a catalyst can be recovered chemically. a catalyst is a substance that will. Catalyst Definition Gcse.
From booytrans.weebly.com
Periodic table catalyst definition chemistry booytrans Catalyst Definition Gcse Hence a catalyst can be recovered chemically. Increases the rate of a reaction. a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. a catalyst is a substance that speeds. Catalyst Definition Gcse.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation ID377097 Catalyst Definition Gcse catalysts increase the rate of chemical reactions by lowering the activation energy. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. Hence a catalyst can be recovered chemically. This means that the mass of a. They do this by changing the reaction pathway. learn about and revise rate of. Catalyst Definition Gcse.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between homogeneous and heterogeneous Catalyst Definition Gcse learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel) study guide. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. They do. Catalyst Definition Gcse.
From exojqwcbb.blob.core.windows.net
Catalyst Chemistry Symbol at Brenda Jones blog Catalyst Definition Gcse a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams.. Catalyst Definition Gcse.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Catalyst Definition Gcse catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. They do this by changing the reaction pathway. a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. a catalyst is a substance that will change the rate of a. Catalyst Definition Gcse.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Definition Gcse a catalyst is a substance that will change the rate of a reaction. Increases the rate of a reaction. They do this by changing the reaction pathway. A catalyst is often used to make a reaction go faster. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. This. Catalyst Definition Gcse.
From dxostlfbq.blob.core.windows.net
Catalytic Definition Physics at Kathleen Wilson blog Catalyst Definition Gcse This means that the mass of a. catalysts increase the rate of chemical reactions by lowering the activation energy. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Is not chemically changed or used up at the end of the. They do this by changing the reaction pathway. Hence. Catalyst Definition Gcse.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalyst Definition Gcse Is not chemically changed or used up at the end of the. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. catalysts increase the rate of chemical reactions by. Catalyst Definition Gcse.
From exobrbkfr.blob.core.windows.net
Catalyst Definition In Your Own Words at Jordan Bryant blog Catalyst Definition Gcse catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel) study guide. Increases the rate of a reaction. This pathway has a lower activation energy,. Hence a catalyst can be recovered chemically. a catalyst is. Catalyst Definition Gcse.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Definition Gcse catalysts increase the rate of chemical reactions by lowering the activation energy. This means that the mass of a. They do this by changing the reaction pathway. A catalyst is often used to make a reaction go faster. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; This pathway. Catalyst Definition Gcse.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Definition Gcse This pathway has a lower activation energy,. Increases the rate of a reaction. learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel) study guide. They do this by changing the reaction pathway. A catalyst is often used to make a reaction go faster. a catalyst is a substance that increases the rate. Catalyst Definition Gcse.
From dxogglyss.blob.core.windows.net
Catalysts Meaning at Betty Thompson blog Catalyst Definition Gcse This means that the mass of a. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. catalysts increase the rate of chemical reactions by lowering the activation energy. Is not chemically changed or used up at the end of the. Increases the rate of a reaction. Hence a. Catalyst Definition Gcse.
From exobfetmk.blob.core.windows.net
Examples Of Catalysts Gcse Chemistry at Colin Aleman blog Catalyst Definition Gcse This means that the mass of a. learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel) study guide. a catalyst is a substance that will change the rate of a reaction. a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process.. Catalyst Definition Gcse.
From www.savemyexams.com
Catalysts AQA GCSE Chemistry Revision Notes 2018 Catalyst Definition Gcse a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. Increases the rate of a reaction. Hence a catalyst can be recovered chemically. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. They do this by changing the. Catalyst Definition Gcse.
From exobfetmk.blob.core.windows.net
Examples Of Catalysts Gcse Chemistry at Colin Aleman blog Catalyst Definition Gcse catalysts increase the rate of chemical reactions by lowering the activation energy. Hence a catalyst can be recovered chemically. A catalyst is often used to make a reaction go faster. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. a catalyst is a substance that will change the rate. Catalyst Definition Gcse.
From exozpzccv.blob.core.windows.net
Catalyst Biology Simple Definition at Lydia Hatcher blog Catalyst Definition Gcse a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. This pathway has a lower activation energy,. Increases the rate of a reaction. learn about and revise rate of. Catalyst Definition Gcse.
From www.slideshare.net
Catalyst Catalyst Definition Gcse catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. Increases the rate of a reaction. This pathway has a lower activation energy,. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; catalysts increase the rate of chemical reactions by lowering. Catalyst Definition Gcse.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID4503154 Catalyst Definition Gcse Hence a catalyst can be recovered chemically. This means that the mass of a. Is not chemically changed or used up at the end of the. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. a catalyst is a substance that will change the rate of a reaction. They do. Catalyst Definition Gcse.
From thechemistrynotes.com
Catalysis Definition, Types, Advantages, Disadvantages Catalyst Definition Gcse This means that the mass of a. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. They do this by changing the reaction pathway. a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. learn about and revise rate. Catalyst Definition Gcse.
From www.slideserve.com
PPT Lesson Five PowerPoint Presentation ID2215771 Catalyst Definition Gcse a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. Hence a catalyst can be recovered chemically. They do this by changing the reaction pathway. a catalyst is a substance that will change the rate of a reaction. a catalyst is a substance that speeds up a. Catalyst Definition Gcse.
From www.youtube.com
GCSE Chemistry Catalysts YouTube Catalyst Definition Gcse They do this by changing the reaction pathway. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. a catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Is not chemically changed or used up at the end of the. . Catalyst Definition Gcse.
From exorbcrky.blob.core.windows.net
Catalyst Meaning Chem at Rick Franks blog Catalyst Definition Gcse Hence a catalyst can be recovered chemically. a catalyst is a substance that will change the rate of a reaction. a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. Is not chemically changed or used up at the end of the. catalysts increase the rate of. Catalyst Definition Gcse.
From gamesmartz.com
Homogeneous Catalyst Definition & Image GameSmartz Catalyst Definition Gcse a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. They do this by changing the reaction pathway. a catalyst is a substance that will change the rate of a reaction. This pathway has a lower activation energy,. Is not chemically changed or used up at the end. Catalyst Definition Gcse.
From www.scribd.com
Catalyst Definition PDF Catalysis Reaction Rate Catalyst Definition Gcse They do this by changing the reaction pathway. learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel) study guide. revision notes on catalysts for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. a catalyst is a substance that speeds up a chemical reaction, but is. Catalyst Definition Gcse.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst Definition Gcse Hence a catalyst can be recovered chemically. Is not chemically changed or used up at the end of the. They do this by changing the reaction pathway. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. This means that the mass of a. Increases the rate of a reaction. A catalyst. Catalyst Definition Gcse.
From www.slideshare.net
Heterogeneous catalysisFundamentals Catalyst Definition Gcse a catalyst is a substance that will change the rate of a reaction. catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. Is not chemically changed or used up at the end of the. learn about and revise rate of reaction with this bbc bitesize gcse combined science (edexcel). Catalyst Definition Gcse.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Definition Gcse a catalyst is a substance that will change the rate of a reaction. This pathway has a lower activation energy,. They do this by changing the reaction pathway. a catalyst is a substance that increases the rate of a chemical reaction, without being used up in the process. learn about and revise rate of reaction with this. Catalyst Definition Gcse.