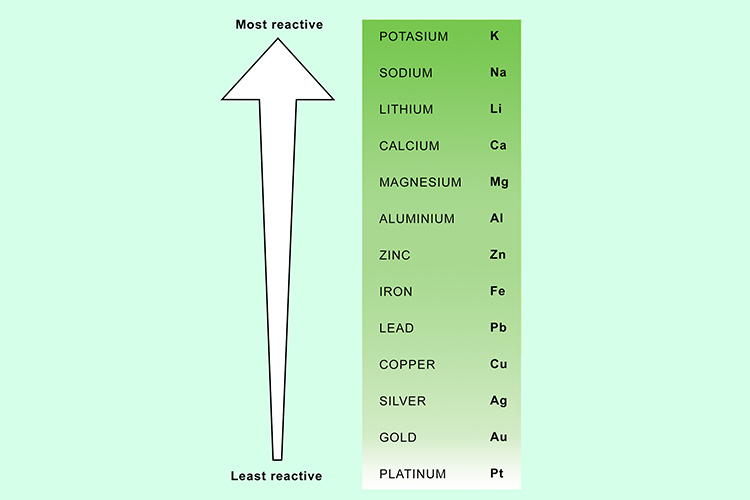
Is Silver More Reactive Than Aluminum . Unreactive metals below hydrogen, such as gold, silver, and copper, do not. 58 rows copper and silver will react with nitric acid; What’s important is keeping in mind the general. reactivity series is a list of metals arranged in decreasing order of their reactivity. More reactive metals displace less reactive metals from. 9 rows the reactivity series ranks metals by how readily they react. the order of reactivity of these four metals with water from most to least reactive is: 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require. particular metals react more with one acid than another, plus temperature plays a role. Most reactive metals are at the. only metals above hydrogen in the reactivity series will react with dilute acids. But because nitric acid is an oxidizing acid, the oxidizing agent is not.
from mammothmemory.net
the order of reactivity of these four metals with water from most to least reactive is: only metals above hydrogen in the reactivity series will react with dilute acids. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require. Unreactive metals below hydrogen, such as gold, silver, and copper, do not. What’s important is keeping in mind the general. But because nitric acid is an oxidizing acid, the oxidizing agent is not. 58 rows copper and silver will react with nitric acid; Most reactive metals are at the. reactivity series is a list of metals arranged in decreasing order of their reactivity. More reactive metals displace less reactive metals from.
More reactive metals displace others in a solution reaction
Is Silver More Reactive Than Aluminum 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require. reactivity series is a list of metals arranged in decreasing order of their reactivity. the order of reactivity of these four metals with water from most to least reactive is: only metals above hydrogen in the reactivity series will react with dilute acids. More reactive metals displace less reactive metals from. But because nitric acid is an oxidizing acid, the oxidizing agent is not. particular metals react more with one acid than another, plus temperature plays a role. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require. 58 rows copper and silver will react with nitric acid; 9 rows the reactivity series ranks metals by how readily they react. What’s important is keeping in mind the general. Most reactive metals are at the. Unreactive metals below hydrogen, such as gold, silver, and copper, do not.
From www.geeksforgeeks.org
Reactivity Series Reactivity of Metals, Features, Tricks Is Silver More Reactive Than Aluminum But because nitric acid is an oxidizing acid, the oxidizing agent is not. More reactive metals displace less reactive metals from. 58 rows copper and silver will react with nitric acid; Unreactive metals below hydrogen, such as gold, silver, and copper, do not. 9 rows the reactivity series ranks metals by how readily they react. reactivity series. Is Silver More Reactive Than Aluminum.
From brainly.in
copper is more reactive than silver,gold and platinum prove with chemical equation Brainly.in Is Silver More Reactive Than Aluminum Unreactive metals below hydrogen, such as gold, silver, and copper, do not. Most reactive metals are at the. the order of reactivity of these four metals with water from most to least reactive is: particular metals react more with one acid than another, plus temperature plays a role. More reactive metals displace less reactive metals from. 58. Is Silver More Reactive Than Aluminum.
From bestweddingblog47.blogspot.com
Which Group Of Metals Is The Most Reactive Is Silver More Reactive Than Aluminum What’s important is keeping in mind the general. More reactive metals displace less reactive metals from. 58 rows copper and silver will react with nitric acid; reactivity series is a list of metals arranged in decreasing order of their reactivity. particular metals react more with one acid than another, plus temperature plays a role. Most reactive metals. Is Silver More Reactive Than Aluminum.
From www.nagwa.com
Question Video Identifying the Most Reactive Metal from a List of Five Metals Nagwa Is Silver More Reactive Than Aluminum reactivity series is a list of metals arranged in decreasing order of their reactivity. particular metals react more with one acid than another, plus temperature plays a role. the order of reactivity of these four metals with water from most to least reactive is: 9 rows the reactivity series ranks metals by how readily they react.. Is Silver More Reactive Than Aluminum.
From www.slideserve.com
PPT CHAPTER 3 METALS AND NON METALS PowerPoint Presentation, free download ID598959 Is Silver More Reactive Than Aluminum More reactive metals displace less reactive metals from. Most reactive metals are at the. Unreactive metals below hydrogen, such as gold, silver, and copper, do not. But because nitric acid is an oxidizing acid, the oxidizing agent is not. What’s important is keeping in mind the general. 9 rows the reactivity series ranks metals by how readily they react.. Is Silver More Reactive Than Aluminum.
From yesdirt.com
Silver vs Aluminum What Are They, And What's the Difference? Yes Dirt Is Silver More Reactive Than Aluminum Most reactive metals are at the. More reactive metals displace less reactive metals from. 58 rows copper and silver will react with nitric acid; What’s important is keeping in mind the general. particular metals react more with one acid than another, plus temperature plays a role. only metals above hydrogen in the reactivity series will react with. Is Silver More Reactive Than Aluminum.
From sciencenotes.org
Activity Series of Metals (Reactivity Series) Is Silver More Reactive Than Aluminum 58 rows copper and silver will react with nitric acid; More reactive metals displace less reactive metals from. reactivity series is a list of metals arranged in decreasing order of their reactivity. What’s important is keeping in mind the general. Unreactive metals below hydrogen, such as gold, silver, and copper, do not. particular metals react more with. Is Silver More Reactive Than Aluminum.
From www.chemedx.org
Using Chemistry to Find that Silver Lining Chemical Education Xchange Is Silver More Reactive Than Aluminum particular metals react more with one acid than another, plus temperature plays a role. But because nitric acid is an oxidizing acid, the oxidizing agent is not. 58 rows copper and silver will react with nitric acid; More reactive metals displace less reactive metals from. 26 rows metals that require the loss of only one electron to. Is Silver More Reactive Than Aluminum.
From anordinary-life.blogspot.com
An Ordinary Life Science Cleaning silver Metal Displacement reaction Is Silver More Reactive Than Aluminum the order of reactivity of these four metals with water from most to least reactive is: Most reactive metals are at the. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require. But because nitric acid is an oxidizing acid, the oxidizing agent is not.. Is Silver More Reactive Than Aluminum.
From getrevising.co.uk
The Reactivity Series Revision Notes in GCSE Chemistry Is Silver More Reactive Than Aluminum 9 rows the reactivity series ranks metals by how readily they react. Unreactive metals below hydrogen, such as gold, silver, and copper, do not. only metals above hydrogen in the reactivity series will react with dilute acids. Most reactive metals are at the. particular metals react more with one acid than another, plus temperature plays a role.. Is Silver More Reactive Than Aluminum.
From brainly.in
how will you show that copper is more reactive than silver Brainly.in Is Silver More Reactive Than Aluminum 9 rows the reactivity series ranks metals by how readily they react. only metals above hydrogen in the reactivity series will react with dilute acids. Unreactive metals below hydrogen, such as gold, silver, and copper, do not. the order of reactivity of these four metals with water from most to least reactive is: But because nitric acid. Is Silver More Reactive Than Aluminum.
From blog.thepipingmart.com
What is Silver Aluminum? Uses and Properties Is Silver More Reactive Than Aluminum particular metals react more with one acid than another, plus temperature plays a role. But because nitric acid is an oxidizing acid, the oxidizing agent is not. 58 rows copper and silver will react with nitric acid; only metals above hydrogen in the reactivity series will react with dilute acids. Unreactive metals below hydrogen, such as gold,. Is Silver More Reactive Than Aluminum.
From www.simplechemconcepts.com
OLevel and IP Pure Chemistry Reactivity Series of Metals Is Silver More Reactive Than Aluminum 58 rows copper and silver will react with nitric acid; 9 rows the reactivity series ranks metals by how readily they react. But because nitric acid is an oxidizing acid, the oxidizing agent is not. reactivity series is a list of metals arranged in decreasing order of their reactivity. Most reactive metals are at the. only. Is Silver More Reactive Than Aluminum.
From www.pw.live
Chemical Reaction and Equation Definition, Formulas, Examples, Notes PW Is Silver More Reactive Than Aluminum 58 rows copper and silver will react with nitric acid; Most reactive metals are at the. Unreactive metals below hydrogen, such as gold, silver, and copper, do not. More reactive metals displace less reactive metals from. 9 rows the reactivity series ranks metals by how readily they react. But because nitric acid is an oxidizing acid, the oxidizing. Is Silver More Reactive Than Aluminum.
From www.adda247.com
Reactivity series of Metals and Non Metals Is Silver More Reactive Than Aluminum Most reactive metals are at the. 9 rows the reactivity series ranks metals by how readily they react. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require. More reactive metals displace less reactive metals from. Unreactive metals below hydrogen, such as gold, silver, and. Is Silver More Reactive Than Aluminum.
From www.slideshare.net
Metals Reactivity Series Is Silver More Reactive Than Aluminum Unreactive metals below hydrogen, such as gold, silver, and copper, do not. reactivity series is a list of metals arranged in decreasing order of their reactivity. particular metals react more with one acid than another, plus temperature plays a role. But because nitric acid is an oxidizing acid, the oxidizing agent is not. Most reactive metals are at. Is Silver More Reactive Than Aluminum.
From www.slideshare.net
Metals Reactivity Series Is Silver More Reactive Than Aluminum particular metals react more with one acid than another, plus temperature plays a role. 58 rows copper and silver will react with nitric acid; only metals above hydrogen in the reactivity series will react with dilute acids. the order of reactivity of these four metals with water from most to least reactive is: 9 rows. Is Silver More Reactive Than Aluminum.
From www.slideshare.net
Metals Is Silver More Reactive Than Aluminum But because nitric acid is an oxidizing acid, the oxidizing agent is not. Most reactive metals are at the. 58 rows copper and silver will react with nitric acid; More reactive metals displace less reactive metals from. Unreactive metals below hydrogen, such as gold, silver, and copper, do not. the order of reactivity of these four metals with. Is Silver More Reactive Than Aluminum.
From www.slideserve.com
PPT Chapter 10 Chemical Reactions and Equations PowerPoint Presentation ID9681335 Is Silver More Reactive Than Aluminum Most reactive metals are at the. only metals above hydrogen in the reactivity series will react with dilute acids. particular metals react more with one acid than another, plus temperature plays a role. What’s important is keeping in mind the general. Unreactive metals below hydrogen, such as gold, silver, and copper, do not. But because nitric acid is. Is Silver More Reactive Than Aluminum.
From polizneuro.weebly.com
Reactivity series of metals polizneuro Is Silver More Reactive Than Aluminum Unreactive metals below hydrogen, such as gold, silver, and copper, do not. reactivity series is a list of metals arranged in decreasing order of their reactivity. particular metals react more with one acid than another, plus temperature plays a role. What’s important is keeping in mind the general. More reactive metals displace less reactive metals from. 9. Is Silver More Reactive Than Aluminum.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Is Silver More Reactive Than Aluminum 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require. More reactive metals displace less reactive metals from. Most reactive metals are at the. What’s important is keeping in mind the general. reactivity series is a list of metals arranged in decreasing order of their. Is Silver More Reactive Than Aluminum.
From www.chegg.com
Solved Tin is a more reactive metal than silver but less Is Silver More Reactive Than Aluminum reactivity series is a list of metals arranged in decreasing order of their reactivity. More reactive metals displace less reactive metals from. 58 rows copper and silver will react with nitric acid; Most reactive metals are at the. What’s important is keeping in mind the general. only metals above hydrogen in the reactivity series will react with. Is Silver More Reactive Than Aluminum.
From www.periodictableprintable.com
Less Reactive Metals In The Periodic Table 2024 Periodic Table Printable Is Silver More Reactive Than Aluminum reactivity series is a list of metals arranged in decreasing order of their reactivity. 9 rows the reactivity series ranks metals by how readily they react. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require. only metals above hydrogen in the reactivity. Is Silver More Reactive Than Aluminum.
From classfullcasandra.z21.web.core.windows.net
Reactivity Chart Periodic Table Is Silver More Reactive Than Aluminum 58 rows copper and silver will react with nitric acid; the order of reactivity of these four metals with water from most to least reactive is: Most reactive metals are at the. 9 rows the reactivity series ranks metals by how readily they react. But because nitric acid is an oxidizing acid, the oxidizing agent is not.. Is Silver More Reactive Than Aluminum.
From mammothmemory.net
More reactive metals displace others in a solution reaction Is Silver More Reactive Than Aluminum particular metals react more with one acid than another, plus temperature plays a role. What’s important is keeping in mind the general. 58 rows copper and silver will react with nitric acid; only metals above hydrogen in the reactivity series will react with dilute acids. the order of reactivity of these four metals with water from. Is Silver More Reactive Than Aluminum.
From www.periodictableprintable.com
What Is The Most Reactive Element In The Periodic Table 2024 Periodic Table Printable Is Silver More Reactive Than Aluminum Unreactive metals below hydrogen, such as gold, silver, and copper, do not. only metals above hydrogen in the reactivity series will react with dilute acids. But because nitric acid is an oxidizing acid, the oxidizing agent is not. What’s important is keeping in mind the general. reactivity series is a list of metals arranged in decreasing order of. Is Silver More Reactive Than Aluminum.
From blog.thepipingmart.com
Silver Foil vs Aluminium Foil What's the Difference Is Silver More Reactive Than Aluminum only metals above hydrogen in the reactivity series will react with dilute acids. More reactive metals displace less reactive metals from. Unreactive metals below hydrogen, such as gold, silver, and copper, do not. But because nitric acid is an oxidizing acid, the oxidizing agent is not. What’s important is keeping in mind the general. particular metals react more. Is Silver More Reactive Than Aluminum.
From facts.net
45 Interesting Aluminum Facts You Never Knew About Is Silver More Reactive Than Aluminum Most reactive metals are at the. the order of reactivity of these four metals with water from most to least reactive is: Unreactive metals below hydrogen, such as gold, silver, and copper, do not. 58 rows copper and silver will react with nitric acid; only metals above hydrogen in the reactivity series will react with dilute acids.. Is Silver More Reactive Than Aluminum.
From blog.thepipingmart.com
Which metal is more reactive iron or zinc Is Silver More Reactive Than Aluminum Unreactive metals below hydrogen, such as gold, silver, and copper, do not. only metals above hydrogen in the reactivity series will react with dilute acids. What’s important is keeping in mind the general. But because nitric acid is an oxidizing acid, the oxidizing agent is not. the order of reactivity of these four metals with water from most. Is Silver More Reactive Than Aluminum.
From quizrapturised.z4.web.core.windows.net
Metals Most Reactive To Least Reactive Is Silver More Reactive Than Aluminum Most reactive metals are at the. 58 rows copper and silver will react with nitric acid; 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require. the order of reactivity of these four metals with water from most to least reactive is: More reactive. Is Silver More Reactive Than Aluminum.
From www.thesciencehive.co.uk
Extraction and uses of metals (GCSE) — the science hive Is Silver More Reactive Than Aluminum particular metals react more with one acid than another, plus temperature plays a role. only metals above hydrogen in the reactivity series will react with dilute acids. Unreactive metals below hydrogen, such as gold, silver, and copper, do not. the order of reactivity of these four metals with water from most to least reactive is: Most reactive. Is Silver More Reactive Than Aluminum.
From www.zephyrus.co.uk
The Reactivity Series Is Silver More Reactive Than Aluminum 9 rows the reactivity series ranks metals by how readily they react. 58 rows copper and silver will react with nitric acid; What’s important is keeping in mind the general. reactivity series is a list of metals arranged in decreasing order of their reactivity. Most reactive metals are at the. 26 rows metals that require the. Is Silver More Reactive Than Aluminum.
From dolfnewsletter.weebly.com
Most reactive metal on periodic table dolfnewsletter Is Silver More Reactive Than Aluminum Unreactive metals below hydrogen, such as gold, silver, and copper, do not. Most reactive metals are at the. the order of reactivity of these four metals with water from most to least reactive is: More reactive metals displace less reactive metals from. 9 rows the reactivity series ranks metals by how readily they react. particular metals react. Is Silver More Reactive Than Aluminum.
From exoyagyrt.blob.core.windows.net
Pan Reactivity Definition at Marian Collins blog Is Silver More Reactive Than Aluminum reactivity series is a list of metals arranged in decreasing order of their reactivity. the order of reactivity of these four metals with water from most to least reactive is: Most reactive metals are at the. particular metals react more with one acid than another, plus temperature plays a role. 26 rows metals that require the. Is Silver More Reactive Than Aluminum.
From mekhi-has-pratt.blogspot.com
Which of the Following Would Be More Reactive Than Magnesium MekhihasPratt Is Silver More Reactive Than Aluminum the order of reactivity of these four metals with water from most to least reactive is: But because nitric acid is an oxidizing acid, the oxidizing agent is not. 9 rows the reactivity series ranks metals by how readily they react. What’s important is keeping in mind the general. 58 rows copper and silver will react with. Is Silver More Reactive Than Aluminum.