Endothermic Reaction Kj . an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). \text{kj}\) of heat is absorbed. An endothermic process does not involve a. \text{mol}\) of carbon dioxide, \(177.8 \: Other chemical reactions release energy in the form of heat, light, or sound. the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from the. \text{mol}\) of calcium carbonate decomposes into \(1 \: Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Reactants + energy (heat) → products. For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium oxide and \(1 \: E n d o t h e r m i c r e a c t i o n.
from www.chegg.com
Other chemical reactions release energy in the form of heat, light, or sound. the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from the. E n d o t h e r m i c r e a c t i o n. For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). \text{mol}\) of carbon dioxide, \(177.8 \: an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. \text{mol}\) of calcium oxide and \(1 \: \text{mol}\) of calcium carbonate decomposes into \(1 \: Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. \text{kj}\) of heat is absorbed.
Solved What is the value of the activation energy for
Endothermic Reaction Kj endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). E n d o t h e r m i c r e a c t i o n. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). An endothermic process does not involve a. Reactants + energy (heat) → products. \text{mol}\) of calcium oxide and \(1 \: the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from the. \text{mol}\) of carbon dioxide, \(177.8 \: \text{kj}\) of heat is absorbed. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium carbonate decomposes into \(1 \: For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Other chemical reactions release energy in the form of heat, light, or sound.
From wiringdbdementiushl.z4.web.core.windows.net
Endothermic And Exothermic Reaction Diagram Endothermic Reaction Kj \text{kj}\) of heat is absorbed. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium carbonate decomposes into \(1 \: E n d o t h e r m i c r e a c t i o n. \text{mol}\) of calcium oxide and \(1 \: Reactants + energy (heat) → products. Other chemical reactions release energy in the. Endothermic Reaction Kj.
From slideplayer.com
Energetics/Thermochemistry ppt download Endothermic Reaction Kj \text{mol}\) of carbon dioxide, \(177.8 \: An endothermic process does not involve a. Reactants + energy (heat) → products. \text{mol}\) of calcium carbonate decomposes into \(1 \: Other chemical reactions release energy in the form of heat, light, or sound. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). \text{mol}\) of. Endothermic Reaction Kj.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Kj Other chemical reactions release energy in the form of heat, light, or sound. \text{mol}\) of calcium carbonate decomposes into \(1 \: E n d o t h e r m i c r e a c t i o n. the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from the. an. Endothermic Reaction Kj.
From slideplayer.com
7.3 Chapter ppt download Endothermic Reaction Kj \text{mol}\) of calcium oxide and \(1 \: an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{kj}\) of heat is absorbed. Reactants + energy (heat) → products.. Endothermic Reaction Kj.
From partdiagramaminabakery5v.z14.web.core.windows.net
Exothermic And Endothermic Diagrams Endothermic Reaction Kj endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). \text{mol}\) of calcium carbonate decomposes into \(1 \: an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. \text{mol}\) of carbon dioxide, \(177.8 \: \text{kj}\) of heat is absorbed. For an endothermic reaction, energy of activation. Endothermic Reaction Kj.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Kj \text{kj}\) of heat is absorbed. the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from the. For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the. Endothermic Reaction Kj.
From www.chegg.com
Solved What is the value of the activation energy for Endothermic Reaction Kj For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). Reactants + energy (heat) → products. \text{mol}\) of calcium oxide and \(1 \: Other chemical reactions release energy in the form of heat, light, or sound. an endothermic reaction occurs when the temperature of an isolated system decreases while. Endothermic Reaction Kj.
From slideplayer.com
Thermodynamics Enthalpy. ppt download Endothermic Reaction Kj Other chemical reactions release energy in the form of heat, light, or sound. \text{mol}\) of carbon dioxide, \(177.8 \: E n d o t h e r m i c r e a c t i o n. \text{kj}\) of heat is absorbed. \text{mol}\) of calcium oxide and \(1 \: \text{mol}\) of calcium carbonate decomposes into \(1 \: An endothermic. Endothermic Reaction Kj.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Kj An endothermic process does not involve a. \text{mol}\) of carbon dioxide, \(177.8 \: \text{kj}\) of heat is absorbed. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. E n d o t h e r m i c r e a c t i o n. the endothermic reaction is a chemical. Endothermic Reaction Kj.
From www.toppr.com
21] Energy of activation of forward reaction an endothermic process is Endothermic Reaction Kj E n d o t h e r m i c r e a c t i o n. Other chemical reactions release energy in the form of heat, light, or sound. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). \text{mol}\) of carbon dioxide, \(177.8 \: For an endothermic reaction,. Endothermic Reaction Kj.
From studymind.co.uk
Endothermic vs Exothermic Reactions (GCSE Chemistry) Study Mind Endothermic Reaction Kj an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. An endothermic process does not involve a. For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of carbon dioxide, \(177.8 \: \text{mol}\) of calcium. Endothermic Reaction Kj.
From inberp.info
Differences between exothermic and endothermic reactions (2023) Endothermic Reaction Kj Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. E n d o t h e r m i c r e a c t i o n. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). an endothermic reaction occurs. Endothermic Reaction Kj.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Kj For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium oxide and \(1 \: \text{mol}\) of carbon dioxide, \(177.8 \: \text{kj}\) of heat is absorbed. An endothermic process does not involve a. Reactants + energy (heat) → products. . Endothermic Reaction Kj.
From www.coursehero.com
[Solved] Classify the reaction endothermic or exothermic and give the Endothermic Reaction Kj an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). \text{mol}\) of carbon dioxide, \(177.8 \: Reactants + energy (heat) → products. \text{mol}\) of calcium carbonate decomposes into \(1 \: the endothermic reaction. Endothermic Reaction Kj.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Reaction Kj For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). \text{mol}\) of calcium carbonate decomposes into \(1 \: An endothermic process does not involve a. E n d o t h e r m i c r e a c t i o n. \text{mol}\) of calcium carbonate decomposes into. Endothermic Reaction Kj.
From www.slideserve.com
PPT Chapter 11 Thermochemistry PowerPoint Presentation, free download Endothermic Reaction Kj Reactants + energy (heat) → products. \text{mol}\) of carbon dioxide, \(177.8 \: \text{mol}\) of calcium oxide and \(1 \: \text{mol}\) of calcium carbonate decomposes into \(1 \: the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from the. an endothermic reaction occurs when the temperature of an isolated system decreases while the. Endothermic Reaction Kj.
From slideplayer.com
Energetics/Thermochemistry ppt download Endothermic Reaction Kj \text{kj}\) of heat is absorbed. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). Other chemical reactions release energy in the form of heat, light, or sound. \text{mol}\) of carbon dioxide, \(177.8 \:. Endothermic Reaction Kj.
From slideplayer.com
Exothermic and Endothermic Chemical Reactions ppt download Endothermic Reaction Kj E n d o t h e r m i c r e a c t i o n. the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from the. \text{mol}\) of calcium oxide and \(1 \: \text{kj}\) of heat is absorbed. an endothermic reaction occurs when the temperature of an isolated. Endothermic Reaction Kj.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction Kj an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. Reactants + energy (heat) → products. \text{mol}\) of calcium carbonate decomposes into \(1 \: the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from the. E n d o t h e r m i c r. Endothermic Reaction Kj.
From slideplayer.com
Thermochemistry Unit 10 Lesson ppt download Endothermic Reaction Kj \text{mol}\) of carbon dioxide, \(177.8 \: An endothermic process does not involve a. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium oxide and \(1 \: Reactants + energy (heat) → products. the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from the. E n d o t h e r. Endothermic Reaction Kj.
From schematicorensano5824i.z19.web.core.windows.net
Exothermic And Endothermic Diagrams Endothermic Reaction Kj \text{mol}\) of calcium oxide and \(1 \: \text{mol}\) of carbon dioxide, \(177.8 \: endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). An endothermic process does not involve a. E n d o t h e r m i c r e a c t i o n. Exothermic reactions may. Endothermic Reaction Kj.
From slideplayer.com
Thermochemistry Chemistry One B. ppt download Endothermic Reaction Kj Other chemical reactions release energy in the form of heat, light, or sound. \text{kj}\) of heat is absorbed. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. \text{mol}\) of carbon dioxide, \(177.8 \: For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol).. Endothermic Reaction Kj.
From slideplayer.com
Chemical Reactions and Energy ppt download Endothermic Reaction Kj an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. \text{mol}\) of calcium carbonate decomposes into \(1 \: Other chemical reactions release energy in the form of heat, light, or sound. An endothermic process does not involve a. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of carbon dioxide, \(177.8 \: endothermic. Endothermic Reaction Kj.
From slideplayer.com
Endothermic and Exothermic Reactions ppt download Endothermic Reaction Kj For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). E n d o t h e r m i c r e a c t i o n. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. Other chemical reactions release energy. Endothermic Reaction Kj.
From mavink.com
Draw An Energy Level Diagram For An Endothermic Reaction Endothermic Reaction Kj Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. An endothermic process does not involve a. \text{mol}\) of calcium carbonate decomposes into \(1 \: an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. endothermic reactions are characterized by positive heat flow (into the. Endothermic Reaction Kj.
From slideplayer.com
Demos. ppt download Endothermic Reaction Kj Other chemical reactions release energy in the form of heat, light, or sound. \text{mol}\) of carbon dioxide, \(177.8 \: an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. E n d o t h e r m i c r e a c t i o n. Exothermic reactions may occur spontaneously and. Endothermic Reaction Kj.
From www.numerade.com
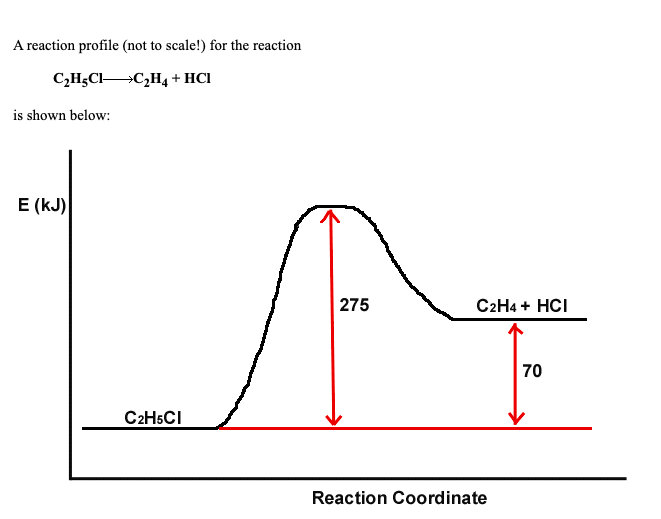
SOLVED A reaction profile (not to scalel) for the reaction 03 NO 02 Endothermic Reaction Kj Other chemical reactions release energy in the form of heat, light, or sound. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). E n d o t h e r m i c r e a c t i o n. For an endothermic reaction, energy of activation is ea and. Endothermic Reaction Kj.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Kj \text{mol}\) of calcium oxide and \(1 \: Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. E n d o t h e r m i c r e a c t i o n. Reactants + energy (heat) → products. the endothermic reaction is a chemical reaction in which. Endothermic Reaction Kj.
From www.youtube.com
For an endothermic reaction, where `Delta H` represents the enthalpy of Endothermic Reaction Kj Other chemical reactions release energy in the form of heat, light, or sound. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of carbon dioxide, \(177.8 \: An endothermic process does not involve a. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Reactants + energy (heat) → products. For an. Endothermic Reaction Kj.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Kj For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both of these in kj/mol). \text{mol}\) of carbon dioxide, \(177.8 \: \text{mol}\) of calcium oxide and \(1 \: Other chemical reactions release energy in the form of heat, light, or sound. \text{mol}\) of calcium carbonate decomposes into \(1 \: E n d o t h. Endothermic Reaction Kj.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction Kj \text{mol}\) of calcium carbonate decomposes into \(1 \: endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). An endothermic process does not involve a. Other chemical reactions release energy in the form of heat, light, or sound. an endothermic reaction occurs when the temperature of an isolated system decreases while. Endothermic Reaction Kj.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Reaction Kj an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. \text{mol}\) of calcium oxide and \(1 \: E n d o t h e r m i c r e a c t i o n. An endothermic process does not involve a. \text{kj}\) of heat is absorbed. Reactants + energy (heat) → products.. Endothermic Reaction Kj.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation Endothermic Reaction Kj An endothermic process does not involve a. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from. Endothermic Reaction Kj.
From www.sarthaks.com
For an endothermic reaction, where `Delta H` represents the enthalpy of Endothermic Reaction Kj the endothermic reaction is a chemical reaction in which the reactants absorb the heat energy from the. E n d o t h e r m i c r e a c t i o n. An endothermic process does not involve a. For an endothermic reaction, energy of activation is ea and enthalpy of reaction is δh (both. Endothermic Reaction Kj.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Kj \text{kj}\) of heat is absorbed. \text{mol}\) of calcium oxide and \(1 \: Other chemical reactions release energy in the form of heat, light, or sound. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of carbon dioxide, \(177.8 \: E n d o t h e r m i c r e a c t i o n. For an. Endothermic Reaction Kj.