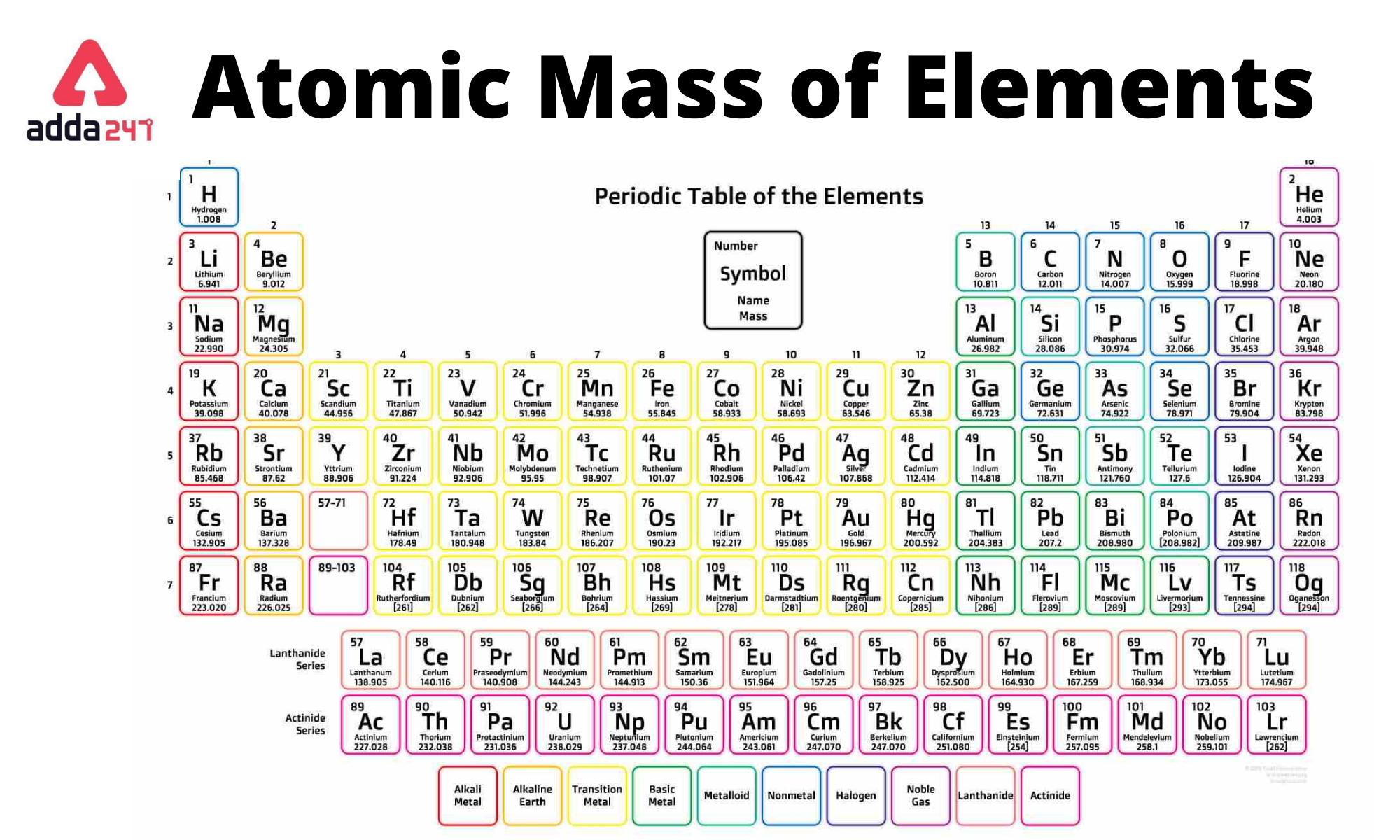
Atomic Mass Of Zinc Class 9 . Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. These ncert questions and answers take. The atomic mass is the mass of an atom. Atomic mass of zinc is 65.409 u. The formula unit mass is same as molecular. The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. It has an atomic weight of 65.38 and a mass. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. The atomic mass or relative isotopic mass refers to. These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning almost immediately. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. The atomic masses are represented in the atomic mass unit (u).
from reviewhomedecor.co
It has an atomic weight of 65.38 and a mass. The atomic mass is the mass of an atom. These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning almost immediately. The formula unit mass is same as molecular. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. The atomic mass or relative isotopic mass refers to. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. The atomic masses are represented in the atomic mass unit (u). These ncert questions and answers take.
First 20 Elements Of The Periodic Table With Atomic Number And Mass Pdf
Atomic Mass Of Zinc Class 9 Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Atomic mass of zinc is 65.409 u. It has an atomic weight of 65.38 and a mass. These ncert questions and answers take. The atomic masses are represented in the atomic mass unit (u). Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. The atomic mass or relative isotopic mass refers to. The atomic mass is the mass of an atom. These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning almost immediately. The formula unit mass is same as molecular. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below.
From www.chegg.com
Solved Part A Atomic Mass of Zinc 1. Mass of zinc _1.0738 Atomic Mass Of Zinc Class 9 It has an atomic weight of 65.38 and a mass. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. The atomic mass is the mass of an atom. The atomic masses are represented in the atomic mass unit (u). These ncert questions. Atomic Mass Of Zinc Class 9.
From www.dreamstime.com
Zinc Chemical Element with First Ionization Energy, Atomic Mass and Atomic Mass Of Zinc Class 9 Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. These ncert questions and answers take. The atomic mass or relative isotopic mass refers to. The atomic mass is the mass of an atom. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic. Atomic Mass Of Zinc Class 9.
From brainly.in
write 30 elements name with consecutive atomic number and their atomic Atomic Mass Of Zinc Class 9 The atomic mass is the mass of an atom. These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning almost immediately. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. The atomic masses are represented in the atomic mass unit (u). The atomic mass or. Atomic Mass Of Zinc Class 9.
From stock.adobe.com
Zinc chemical element with first ionization energy, atomic mass and Atomic Mass Of Zinc Class 9 The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. These ncert questions and answers take. The atomic mass or relative isotopic mass refers to. The atomic mass is the mass of an atom. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic. Atomic Mass Of Zinc Class 9.
From www.youtube.com
Class 9 Questions Of Average Atomic Mass Class 9 Chemistry // Class 9 Atomic Mass Of Zinc Class 9 It has an atomic weight of 65.38 and a mass. The atomic mass or relative isotopic mass refers to. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. The atomic mass is the mass of an atom. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Atomic mass of. Atomic Mass Of Zinc Class 9.
From www.vedantu.com
Zinc Learn Definition, Properties and Facts Atomic Mass Of Zinc Class 9 The formula unit mass is same as molecular. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. The atomic mass or relative isotopic mass refers to. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. Atomic mass of all elements. Atomic Mass Of Zinc Class 9.
From www.toppr.com
Calculate the formula unit masses of ZnO, Na_2O, K_2CO_3, given atomic Atomic Mass Of Zinc Class 9 Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. These ncert questions and answers take. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. Atomic mass of zinc is 65.409 u. The atomic mass is the mass of. Atomic Mass Of Zinc Class 9.
From www.teachoo.com
Definition, How to caluclate Atomic mass Teachoo Science Atomic Mass Of Zinc Class 9 The atomic mass is the mass of an atom. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. The atomic mass or relative isotopic mass refers to. The atomic masses are represented in the atomic mass unit (u). Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. Atomic mass of all. Atomic Mass Of Zinc Class 9.
From www.britannica.com
zinc Properties, Uses, & Facts Britannica Atomic Mass Of Zinc Class 9 It has an atomic weight of 65.38 and a mass. The atomic mass is the mass of an atom. These ncert questions and answers take. These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning almost immediately. The atomic masses are represented in the atomic mass unit (u). The atomic mass or relative isotopic. Atomic Mass Of Zinc Class 9.
From reviewhomedecor.co
First 20 Elements Of The Periodic Table With Atomic Number And Mass Pdf Atomic Mass Of Zinc Class 9 Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. Atomic mass of zinc is 65.409 u. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. It has an atomic weight of 65.38 and a mass. The atomic masses are represented in the atomic mass unit (u). The molecular mass. Atomic Mass Of Zinc Class 9.
From alquilercastilloshinchables.info
7 Images Periodic Table With Names And Atomic Mass Number Valency And Atomic Mass Of Zinc Class 9 Atomic mass of zinc is 65.409 u. The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. The atomic mass is the mass of an atom. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. The atomic mass or relative isotopic mass. Atomic Mass Of Zinc Class 9.
From blogcomputing131.weebly.com
Atomic Mass Of Zinc Atomic Mass Of Zinc Class 9 These ncert questions and answers take. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. The atomic masses are represented in the atomic mass unit (u). Atomic mass of zinc is 65.409 u. The formula unit mass is same as molecular. The molecular mass of an element can be calculated by. Atomic Mass Of Zinc Class 9.
From www.youtube.com
Relative Atomic Mass and Atomic Mass Unit (amu) Fundamental of Atomic Mass Of Zinc Class 9 Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. These ncert questions and answers take. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. The formula unit mass is same as molecular.. Atomic Mass Of Zinc Class 9.
From mdcpgulvwy.blogspot.com
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio Atomic Mass Of Zinc Class 9 Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. The atomic mass or relative isotopic mass refers to. Given below is a table. Atomic Mass Of Zinc Class 9.
From www.youtube.com
A 7 36 g sample of copper is contaminated with an additional 0 51 g of Atomic Mass Of Zinc Class 9 Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. These. Atomic Mass Of Zinc Class 9.
From officialbruinsshop.com
Periodic Table Of Elements With Atomic Mass And Valency Bruin Blog Atomic Mass Of Zinc Class 9 The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning almost immediately. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. The formula unit. Atomic Mass Of Zinc Class 9.
From www.youtube.com
ATOMIC MASS OF ZINC YouTube Atomic Mass Of Zinc Class 9 Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. Atomic mass of zinc is. Atomic Mass Of Zinc Class 9.
From stock.adobe.com
Zinc Zn Chemical Element vector illustration diagram, with atomic Atomic Mass Of Zinc Class 9 The formula unit mass is same as molecular. These ncert questions and answers take. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Atomic mass of zinc is 65.409 u. It has an atomic weight of 65.38 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos.. Atomic Mass Of Zinc Class 9.
From www.nagwa.com
Question Video Calculating the Mass of Zinc Formed in a Cell during a Atomic Mass Of Zinc Class 9 It has an atomic weight of 65.38 and a mass. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. Atomic mass of zinc is 65.409 u. These ncert questions and answers take. These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning almost immediately. The. Atomic Mass Of Zinc Class 9.
From www.youtube.com
What is the number of zinc atoms in a piece of zinc weighing `10 g Atomic Mass Of Zinc Class 9 Atomic mass of zinc is 65.409 u. The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning. Atomic Mass Of Zinc Class 9.
From www.shutterstock.com
Zinc Atomic Structure Has Atomic Number เวกเตอร์สต็อก (ปลอดค่า Atomic Mass Of Zinc Class 9 The atomic mass is the mass of an atom. Atomic mass of zinc is 65.409 u. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. The molecular mass of. Atomic Mass Of Zinc Class 9.
From brainly.in
the atomic mass of zinc is 65.4. a.m.u find no of moles in 10.8 g zinc Atomic Mass Of Zinc Class 9 Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. These ncert questions and answers take. Atomic mass of zinc is 65.409 u. The atomic mass is the mass of an atom. The atomic masses are represented in the atomic mass unit (u). Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. Given. Atomic Mass Of Zinc Class 9.
From material-properties.org
Zinc Periodic Table and Atomic Properties Atomic Mass Of Zinc Class 9 The atomic mass or relative isotopic mass refers to. It has an atomic weight of 65.38 and a mass. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning almost immediately. The molecular mass. Atomic Mass Of Zinc Class 9.
From www.animalia-life.club
Modern Periodic Table With Atomic Mass And Atomic Number Atomic Mass Of Zinc Class 9 The atomic masses are represented in the atomic mass unit (u). These ncert questions and answers take. Atomic mass of zinc is 65.409 u. It has an atomic weight of 65.38 and a mass. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. Zinc is the 30th element in. Atomic Mass Of Zinc Class 9.
From scoop.eduncle.com
Ex the atomic mass of the zinc isotope azn is 63.9294. compare its Atomic Mass Of Zinc Class 9 The atomic masses are represented in the atomic mass unit (u). Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. It has an atomic weight of 65.38 and a mass. The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. Sources, facts,. Atomic Mass Of Zinc Class 9.
From periodic-table--1.blogspot.com
68 PERIODIC TABLE ZINC ATOMIC MASS, PERIODIC MASS ZINC TABLE ATOMIC Atomic Mass Of Zinc Class 9 Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. Atomic mass of zinc is 65.409 u. The atomic mass or relative isotopic mass refers to. The molecular mass of an element can. Atomic Mass Of Zinc Class 9.
From alumnos.planeaciondidactica.cucea.udg.mx
Is that right the mass number of zinc is 98 and the atomic number is 30 Atomic Mass Of Zinc Class 9 Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. Given below is a table that lists the first 30 elements based on atomic. Atomic Mass Of Zinc Class 9.
From stock.adobe.com
Zinc Zn Transition metal Chemical Element vector illustration diagram Atomic Mass Of Zinc Class 9 Atomic mass of zinc is 65.409 u. These ncert questions and answers take. The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. The atomic masses are represented in the atomic. Atomic Mass Of Zinc Class 9.
From www.slideserve.com
PPT Chapter 4 Atomic Structure PowerPoint Presentation, free download Atomic Mass Of Zinc Class 9 Atomic mass of zinc is 65.409 u. These ncert questions and answers take. The formula unit mass is same as molecular. It has an atomic weight of 65.38 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30.. Atomic Mass Of Zinc Class 9.
From www.studocu.com
Atomic Mass and Atomic Number Worksheet Key Studocu Atomic Mass Of Zinc Class 9 These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning almost immediately. These ncert questions and answers take. The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below.. Atomic Mass Of Zinc Class 9.
From www.alamy.com
Zn Zinc Chemical Element Periodic Table. Single vector illustration Atomic Mass Of Zinc Class 9 Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. The atomic mass is the mass of an atom. These chapter 3 class 9 science ncert solutions will allow the students to. Atomic Mass Of Zinc Class 9.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Atomic Mass Of Zinc Class 9 Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. These ncert questions and answers take. The molecular mass of an element can be calculated by adding the atomic masses of each of its constituents.. Atomic Mass Of Zinc Class 9.
From pubchem.ncbi.nlm.nih.gov
Atomic Mass Periodic Table of Elements PubChem Atomic Mass Of Zinc Class 9 It has an atomic weight of 65.38 and a mass. The atomic mass is the mass of an atom. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. The atomic mass or relative isotopic mass refers to. The atomic masses are represented in the atomic mass unit (u). Sources, facts, uses, scarcity. Atomic Mass Of Zinc Class 9.
From askfilo.com
How to learn Atomic Masses? Atomic mass of common elements List H=1He=4.. Atomic Mass Of Zinc Class 9 Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. It has an atomic weight of 65.38 and a mass. These chapter 3 class 9 science ncert solutions will allow the students to evaluate their learning almost immediately. The atomic masses are represented in the atomic mass unit (u). Atomic. Atomic Mass Of Zinc Class 9.
From quizzlibmisty.z21.web.core.windows.net
How To Find The Atomic Mass Atomic Mass Of Zinc Class 9 It has an atomic weight of 65.38 and a mass. The atomic mass or relative isotopic mass refers to. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. Calculate formula unit mass of naci (atomic mass of na = 23 u, cl = 35.5 u) answer. These chapter 3. Atomic Mass Of Zinc Class 9.