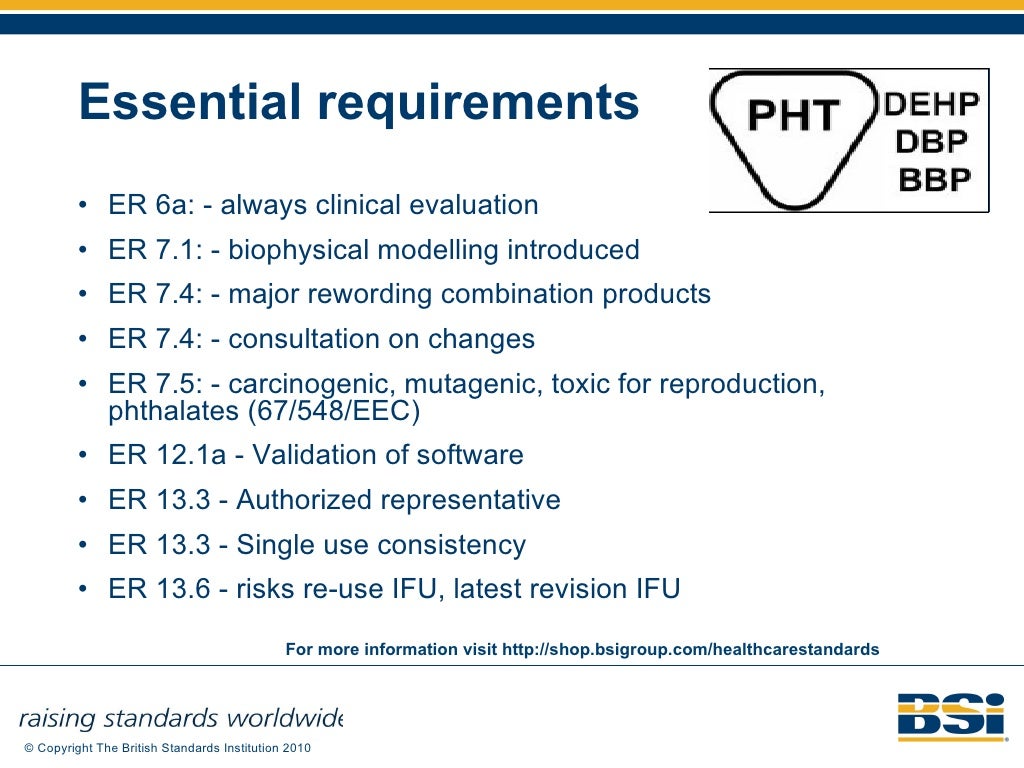
What Is The Eu Medical Device Directive . It updates the rules on. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. What is the aim of the regulation? regulation (eu) 2017/745 on medical devices. medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal.
from www.slideshare.net
In the european union (eu) they must undergo a. regulation (eu) 2017/745 on medical devices. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. What is the aim of the regulation? where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. It updates the rules on. medical devices are products or equipment intended for a medical purpose. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal.
EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update…
What Is The Eu Medical Device Directive medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. In the european union (eu) they must undergo a. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. medical devices are products or equipment intended for a medical purpose. It updates the rules on. What is the aim of the regulation? regulation (eu) 2017/745 on medical devices.
From www.capgemini.com
European Union Medical Device Directive (MDD) to Medical Device What Is The Eu Medical Device Directive What is the aim of the regulation? regulation (eu) 2017/745 on medical devices. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. It updates the rules on. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. regulation (eu) 2017/745. What Is The Eu Medical Device Directive.
From www.stendard.io
6 Major Implementations in the EU Medical Devices Regulation (MDR What Is The Eu Medical Device Directive regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. medical devices are products or equipment intended for a medical purpose. What is the aim of the regulation? where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec,. What Is The Eu Medical Device Directive.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX What Is The Eu Medical Device Directive In the european union (eu) they must undergo a. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted. What Is The Eu Medical Device Directive.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 What Is The Eu Medical Device Directive What is the aim of the regulation? regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. It updates the rules on. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. In the european union (eu) they. What Is The Eu Medical Device Directive.
From www.auxergo.com
The Interactive Guide Under The New EU Regulations on Medical Devices What Is The Eu Medical Device Directive the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. What is the aim of the regulation? regulation (eu) 2017/745 on medical devices. It updates the rules on. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. where a device. What Is The Eu Medical Device Directive.
From www.slideshare.net
EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update… What Is The Eu Medical Device Directive regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a. regulation (eu) 2017/745 on medical devices. It updates the rules on. What is the aim of the regulation? the medical device regulation (mdr), which was adopted in april. What Is The Eu Medical Device Directive.
From www.slideserve.com
PPT Research Governance PowerPoint Presentation, free download ID What Is The Eu Medical Device Directive where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. What is the aim of the regulation? In the european union (eu) they must undergo a. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. It updates. What Is The Eu Medical Device Directive.
From www.slideshare.net
EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update… What Is The Eu Medical Device Directive regulation (eu) 2017/745 on medical devices. It updates the rules on. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. What is the aim of the regulation? where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec,. What Is The Eu Medical Device Directive.
From studylib.net
Impact of EU Medical Device Directive on Medical What Is The Eu Medical Device Directive regulation (eu) 2017/745 on medical devices. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. It updates the rules on. medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. What Is The Eu Medical Device Directive.
From kuaforasistani.com
EU MDR everything you need to know about Medical Device Regulation (2022) What Is The Eu Medical Device Directive where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a. regulation (eu) 2017/745 on medical devices. the medical device regulation (mdr), which was adopted in april. What Is The Eu Medical Device Directive.
From www.slideserve.com
PPT European Union Medical Device Directive (MDD) to Medical Device What Is The Eu Medical Device Directive where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. What is the aim of the regulation? the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. It updates the rules on. In the european union (eu) they must undergo a. regulation. What Is The Eu Medical Device Directive.
From www.slideshare.net
European Union (EU) Medical Device Directive (MDD) Changes Industry What Is The Eu Medical Device Directive medical devices are products or equipment intended for a medical purpose. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. It updates the rules on. What. What Is The Eu Medical Device Directive.
From www.linkedin.com
The EU Medical Device Directive Why, What, and When What Is The Eu Medical Device Directive where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament and of the. What Is The Eu Medical Device Directive.
From exactmm.com
EU Medical Device Directive 9342EEC (page 1).. Exact Medical What Is The Eu Medical Device Directive the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. medical devices are products or equipment intended for a medical purpose. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. It updates the rules on. regulation (eu) 2017/745 on medical. What Is The Eu Medical Device Directive.
From www.motaword.com
EU Medical Device Regulation What Do You Need To Know? What Is The Eu Medical Device Directive What is the aim of the regulation? where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. regulation (eu) 2017/745 on medical devices. medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a. regulation (eu) 2017/745. What Is The Eu Medical Device Directive.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I What Is The Eu Medical Device Directive In the european union (eu) they must undergo a. It updates the rules on. medical devices are products or equipment intended for a medical purpose. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 on medical devices. where a device is intended to administer a medicinal product. What Is The Eu Medical Device Directive.
From www.slideshare.net
EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update… What Is The Eu Medical Device Directive where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. regulation (eu) 2017/745 on medical devices. In the european union (eu) they must undergo a. It updates the rules on. medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the. What Is The Eu Medical Device Directive.
From blog.cosmotrace.com
EU Medical Devices Regulations What Is The Eu Medical Device Directive regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. It updates. What Is The Eu Medical Device Directive.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) What Is The Eu Medical Device Directive What is the aim of the regulation? In the european union (eu) they must undergo a. It updates the rules on. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. medical devices are products or equipment intended for a medical purpose. where a device is intended to administer a medicinal product. What Is The Eu Medical Device Directive.
From exactmm.com
EUMedicalDeviceDirective9342EECpage2.. Exact Medical What Is The Eu Medical Device Directive In the european union (eu) they must undergo a. regulation (eu) 2017/745 on medical devices. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. where a device is intended to. What Is The Eu Medical Device Directive.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is The Eu Medical Device Directive medical devices are products or equipment intended for a medical purpose. What is the aim of the regulation? It updates the rules on. In the european union (eu) they must undergo a. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 on medical devices. where a device. What Is The Eu Medical Device Directive.
From issuu.com
Selling Medical Devices in the EUMedical Device Directive, CE & FDA by What Is The Eu Medical Device Directive regulation (eu) 2017/745 on medical devices. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. What is the aim of the regulation? In the european union (eu) they must undergo a. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal.. What Is The Eu Medical Device Directive.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog What Is The Eu Medical Device Directive In the european union (eu) they must undergo a. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 on medical devices. medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017. What Is The Eu Medical Device Directive.
From www.presentationeze.com
Differences between Australian & EU Medical Device Regulation What Is The Eu Medical Device Directive regulation (eu) 2017/745 on medical devices. medical devices are products or equipment intended for a medical purpose. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. In the european union (eu) they must undergo a. It updates the rules on. the medical device regulation (mdr),. What Is The Eu Medical Device Directive.
From www.behringer.it
STATEMENT TRANSITION FROM THE MDD 93/42/EEC DIRECTIVE TO THE EU What Is The Eu Medical Device Directive It updates the rules on. In the european union (eu) they must undergo a. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. medical devices are products or equipment intended for a medical purpose. where a device is intended to administer a medicinal product within the meaning of article 1 of. What Is The Eu Medical Device Directive.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is The Eu Medical Device Directive the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. In the. What Is The Eu Medical Device Directive.
From www.email.motaword.com
EU Medical Device Regulation What Do You Need To Know? What Is The Eu Medical Device Directive It updates the rules on. What is the aim of the regulation? regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a. where a device is intended to administer a medicinal product within the meaning of article 1 of. What Is The Eu Medical Device Directive.
From www.scribd.com
EU Guide Medical Device Directive Medical Device Medical Specialties What Is The Eu Medical Device Directive medical devices are products or equipment intended for a medical purpose. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. regulation (eu) 2017/745 on medical devices. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. What Is The Eu Medical Device Directive.
From www.presentationeze.com
Medical Devices Directive (MDD) 93/42/EEC European What Is The Eu Medical Device Directive medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a. What is the aim of the regulation? the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 on medical devices. where a device is intended to administer a. What Is The Eu Medical Device Directive.
From www2.deloitte.com
The new European Union medical devices regulation Deloitte Life What Is The Eu Medical Device Directive regulation (eu) 2017/745 on medical devices. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. It updates the rules on. In the european union (eu) they must undergo a. medical devices are products or equipment intended for a medical purpose. the medical device regulation (mdr),. What Is The Eu Medical Device Directive.
From www.regulatoryglobe.com
EU MDR implementation guide for medical devices MDCG What Is The Eu Medical Device Directive What is the aim of the regulation? regulation (eu) 2017/745 on medical devices. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. It updates the rules on. In the european union (eu) they must undergo a. where a device is intended to administer a medicinal product. What Is The Eu Medical Device Directive.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF What Is The Eu Medical Device Directive In the european union (eu) they must undergo a. medical devices are products or equipment intended for a medical purpose. where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. What is. What Is The Eu Medical Device Directive.
From doctorvisit.netlify.app
New medical device directive doctorvisit What Is The Eu Medical Device Directive where a device is intended to administer a medicinal product within the meaning of article 1 of directive 65/65/eec, that. regulation (eu) 2017/745 on medical devices. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. What is the aim of the regulation? the medical device. What Is The Eu Medical Device Directive.
From www.slideshare.net
EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update… What Is The Eu Medical Device Directive medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. What is the aim of the regulation? regulation (eu) 2017/745 on medical devices. It updates the rules on. where a device. What Is The Eu Medical Device Directive.
From medtechintelligence.com
New EU MDR Regulations and Revamp of the Medical Device Directive What Is The Eu Medical Device Directive medical devices are products or equipment intended for a medical purpose. It updates the rules on. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. What is the aim of the regulation? In the european union (eu) they must undergo a. where a device is intended. What Is The Eu Medical Device Directive.