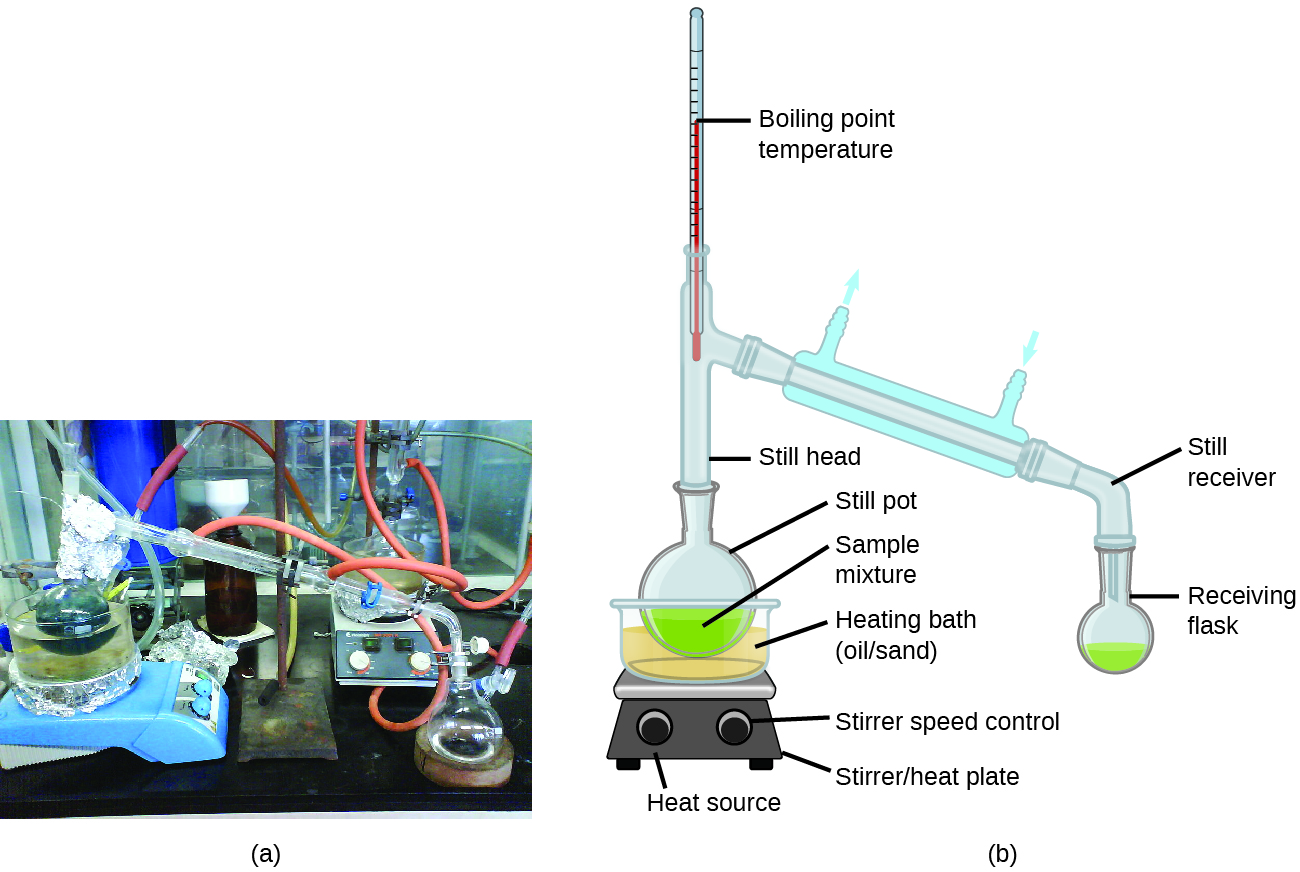
Distillation System In Lab . Apply the heat source to the distilling flask. Record the temperature where liquid is. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Turn on the condenser water. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. A fraction distillation can also be used. Determine the temperature and concentration profiles of the. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Operate the distillation column at total reflux. It can remove impurities, including germs,. Collect distillate at a rate of 1 drop per second. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points.
from philschatz.com
A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. Operate the distillation column at total reflux. Apply the heat source to the distilling flask. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Collect distillate at a rate of 1 drop per second. Record the temperature where liquid is. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Turn on the condenser water. Determine the temperature and concentration profiles of the.
Colligative Properties · Chemistry
Distillation System In Lab A fraction distillation can also be used. Turn on the condenser water. Record the temperature where liquid is. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Determine the temperature and concentration profiles of the. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. It can remove impurities, including germs,. Operate the distillation column at total reflux. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. A fraction distillation can also be used.
From www.labtex.co.uk
Hybrid distillation systems Pope Laboratory Equipment Distillation System In Lab Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A fraction distillation can also be used. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points.. Distillation System In Lab.
From www.coursehero.com
[Solved] Organic Chemistry Laboratory Activity Data/Procedure for Distillation System In Lab Collect distillate at a rate of 1 drop per second. A fraction distillation can also be used. Operate the distillation column at total reflux. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup.. Distillation System In Lab.
From chem.libretexts.org
1A.3 Classifying Matter Chemistry LibreTexts Distillation System In Lab Apply the heat source to the distilling flask. Turn on the condenser water. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. A fraction distillation can also be used. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. It can remove impurities,. Distillation System In Lab.
From kedayq.en.made-in-china.com
Lab Molecular Distillation System Short Path Distillation China Short Distillation System In Lab Determine the temperature and concentration profiles of the. Record the temperature where liquid is. A fraction distillation can also be used. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Distillation is a purification. Distillation System In Lab.
From microbenotes.com
Water Distiller Principle, Parts, Types, Uses, Examples Distillation System In Lab Operate the distillation column at total reflux. Collect distillate at a rate of 1 drop per second. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. A fraction distillation can also be used. It. Distillation System In Lab.
From www.vecteezy.com
Spherical sothlet distillation apparatus diagram for experiment setup Distillation System In Lab A fraction distillation can also be used. It can remove impurities, including germs,. Collect distillate at a rate of 1 drop per second. Operate the distillation column at total reflux. Determine the temperature and concentration profiles of the. Apply the heat source to the distilling flask. Distillation is a purification method for liquids, and can separate components of a mixture. Distillation System In Lab.
From www.simplepharmanotes.com
Distillation under reduced pressure / Vacuum Distillation. Distillation System In Lab Determine the temperature and concentration profiles of the. Operate the distillation column at total reflux. Apply the heat source to the distilling flask. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying. Distillation System In Lab.
From www.thoughtco.com
What Is Distillation? Principles and Uses Distillation System In Lab A fraction distillation can also be used. It can remove impurities, including germs,. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. Apply the heat source to the distilling flask. Distillation is a purification method for liquids, and can separate components of a mixture if they have. Distillation System In Lab.
From tnlab.com
Short Path Distillation System 20 Liter TN LAB Supply Distillation System In Lab Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Determine the temperature and concentration profiles of the. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. A vacuum distillation apparatus is shown in figure 5.50,. Distillation System In Lab.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Distillation System In Lab In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. Operate the distillation column at total reflux. A fraction distillation can also be used. Apply the heat source to the distilling flask. Record the temperature where liquid is. Turn on the condenser water. It can remove impurities, including. Distillation System In Lab.
From www.labrotovap.com
What are the different types of distillation process? Lab Instrument Distillation System In Lab Collect distillate at a rate of 1 drop per second. A fraction distillation can also be used. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Determine the temperature and concentration profiles of the.. Distillation System In Lab.
From glossary.periodni.com
Chemistry Glossary Search results for 'distillation' Distillation System In Lab Turn on the condenser water. Determine the temperature and concentration profiles of the. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Record the temperature where liquid is. Collect distillate at a rate of 1 drop per second. Distillation is a purification method for liquids, and can separate components of a mixture if they have. Distillation System In Lab.
From tnlab.com
Short Path Distillation System 20 Liter TN LAB Supply Distillation System In Lab Collect distillate at a rate of 1 drop per second. Turn on the condenser water. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. In this lab tutorial, we discuss simple distillation, including its. Distillation System In Lab.
From c1d1labs.org
C1D1 Labs Distillation Equipment Distillation System In Lab Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Operate the distillation column at total reflux. Apply the heat source to the distilling flask. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. A vacuum. Distillation System In Lab.
From www.labrotovap.com
The Vacuum Distillation Methods for Cannabis Extraction Process Lab Distillation System In Lab Collect distillate at a rate of 1 drop per second. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. A fraction distillation can also be used. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids.. Distillation System In Lab.
From www.newater.com
Professional Distilled Water Machine for Lab SupplierNEWater Distillation System In Lab Operate the distillation column at total reflux. Collect distillate at a rate of 1 drop per second. Turn on the condenser water. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. It can remove impurities, including germs,. A fraction distillation can also be used. Record the temperature where. Distillation System In Lab.
From ceahjckh.blob.core.windows.net
How To Make Distilled Water In Chemistry Lab at Byron Taylor blog Distillation System In Lab Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Operate the distillation column at total reflux. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. A fraction distillation can also be used. Record the temperature. Distillation System In Lab.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Distillation System In Lab Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Operate the distillation column at total reflux. A fraction distillation can also be used. Record the temperature where liquid is. Turn on the condenser water. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup.. Distillation System In Lab.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Distillation System In Lab Turn on the condenser water. Determine the temperature and concentration profiles of the. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Operate the distillation column at total reflux. It can remove impurities, including germs,. Distillation is one of the oldest and still most common methods for both. Distillation System In Lab.
From jupiter.plymouth.edu
DISTILLATION APPARATUS Distillation System In Lab Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. Turn on the condenser water. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. Distillation is a purification method for liquids, and can separate components of a mixture if. Distillation System In Lab.
From trimscene.com
Lab Society 20L Short Path Distillation Kit Trim Scene Distillation System In Lab A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Turn on the condenser water. Apply the heat source to the distilling flask. Determine the temperature and concentration profiles of the. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. It can remove impurities,. Distillation System In Lab.
From www.youtube.com
how to prepare distilled water distilled water preparation in Distillation System In Lab Operate the distillation column at total reflux. Turn on the condenser water. Determine the temperature and concentration profiles of the. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. Collect distillate at a rate of 1 drop per second. Record the temperature where liquid is. Distillation is. Distillation System In Lab.
From en.wikipedia.org
Distillation Wikipedia Distillation System In Lab Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Turn on the condenser water. It can remove impurities, including germs,. Record the temperature where liquid is. Operate the distillation column at total reflux. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its. Distillation System In Lab.
From dxonzryyy.blob.core.windows.net
How To Make Your Own Distillation Apparatus at Jonathan Carbone blog Distillation System In Lab It can remove impurities, including germs,. Turn on the condenser water. Operate the distillation column at total reflux. A fraction distillation can also be used. Apply the heat source to the distilling flask. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Collect distillate at a rate of 1 drop per second. Distillation is a. Distillation System In Lab.
From www.rotovap-wellknown.com
md60labshortpathmoleculardistillation Molecular distillation Distillation System In Lab Turn on the condenser water. Apply the heat source to the distilling flask. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Determine the temperature and concentration profiles of the. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. A fraction distillation can. Distillation System In Lab.
From labomiz.com
Dual distilled water distiller MDWD1C Labomiz Laboratory Equipment Distillation System In Lab Turn on the condenser water. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. Record the temperature where liquid is. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Determine the temperature and concentration profiles. Distillation System In Lab.
From chem.libretexts.org
5.2C StepbyStep Procedures Chemistry LibreTexts Distillation System In Lab Apply the heat source to the distilling flask. It can remove impurities, including germs,. A fraction distillation can also be used. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Turn on the condenser water. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. Distillation System In Lab.
From labassociates.com
Water distillation for PTC seed labs Lab Associates Distillation System In Lab A fraction distillation can also be used. Determine the temperature and concentration profiles of the. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Turn on the condenser water. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Apply the heat source to. Distillation System In Lab.
From www.southernlabware.com
Distillation Kit with Graham Condenser, Complete, 1000ml Southern Labware Distillation System In Lab It can remove impurities, including germs,. Operate the distillation column at total reflux. Collect distillate at a rate of 1 drop per second. Record the temperature where liquid is. Apply the heat source to the distilling flask. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. Distillation. Distillation System In Lab.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Distillation System In Lab A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. It can remove impurities, including germs,. Apply the heat source to the distilling flask. Determine the temperature and concentration profiles of the. Operate the distillation column at total reflux. Record the temperature where liquid is. Collect distillate at a rate of 1 drop per second. Distillation. Distillation System In Lab.
From philschatz.com
Colligative Properties · Chemistry Distillation System In Lab Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Apply the heat source to the distilling flask. A fraction distillation can also be used. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. It can. Distillation System In Lab.
From www.indiamart.com
Horizontal Water Distillation Unit , Single, Lab Tech Scientific Works Distillation System In Lab Record the temperature where liquid is. Turn on the condenser water. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. Collect distillate at a rate of 1 drop per second. A fraction distillation can also be used. Apply the heat source to the distilling flask. A vacuum. Distillation System In Lab.
From www.shutterstock.com
Vektor Stok Simple Distillation Laboratory Set Separation Homogeneous Distillation System In Lab Record the temperature where liquid is. Collect distillate at a rate of 1 drop per second. Determine the temperature and concentration profiles of the. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Apply the heat source to the distilling flask. Operate the distillation column at total reflux.. Distillation System In Lab.
From ceahjckh.blob.core.windows.net
How To Make Distilled Water In Chemistry Lab at Byron Taylor blog Distillation System In Lab Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Operate the distillation column at total reflux. It can remove impurities, including germs,. A vacuum distillation apparatus is. Distillation System In Lab.
From www.biophlox.com
Buy Genist International Water Distillation Unit get price for lab Distillation System In Lab A fraction distillation can also be used. In this lab tutorial, we discuss simple distillation, including its distinction from fractional distillation, its underlying physical chemistry, and its basic setup. Apply the heat source to the distilling flask. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. A vacuum. Distillation System In Lab.