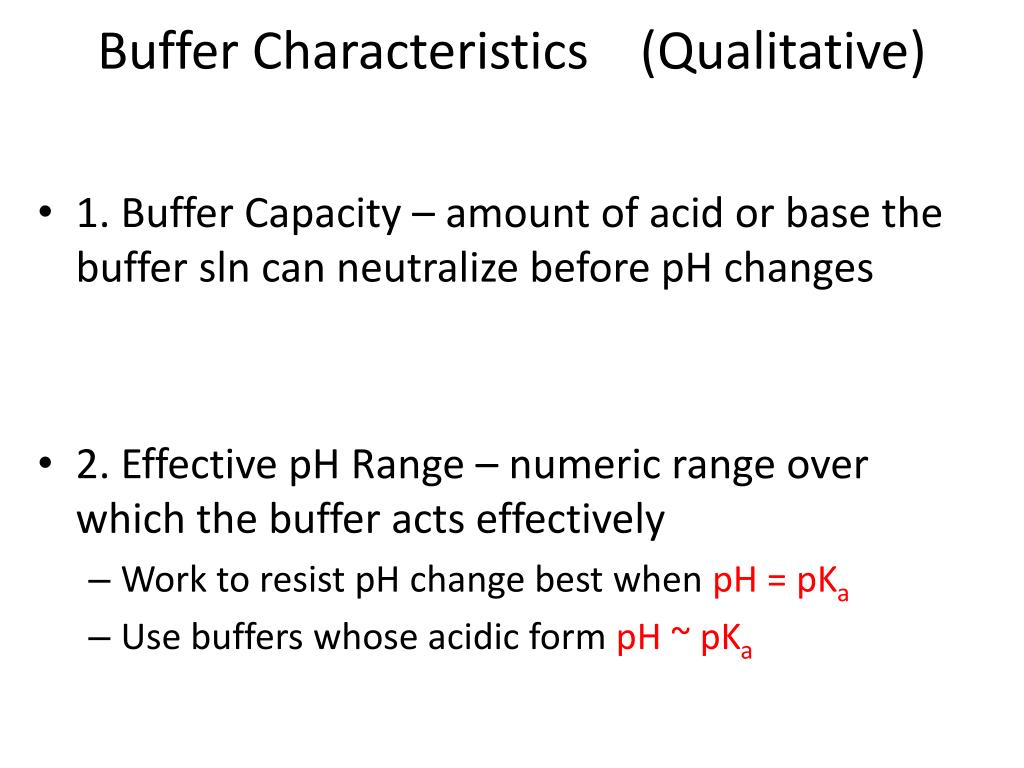
Relationship Between Ph And Buffer Capacity . Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. Only the concentration of h + and. ph and buffers defined. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. A buffer is a solution that resists a significant change. therefore, to calculate buffer capacity, we use the following formula: buffers • biological systems use buffers to maintain ph. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes.
from www.slideserve.com
Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. therefore, to calculate buffer capacity, we use the following formula: buffers • biological systems use buffers to maintain ph. ph and buffers defined. A buffer is a solution that resists a significant change. Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Only the concentration of h + and.
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint
Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. A buffer is a solution that resists a significant change. Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. Only the concentration of h + and. therefore, to calculate buffer capacity, we use the following formula: ph and buffers defined. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. buffers • biological systems use buffers to maintain ph. Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the.
From www.differencebetween.com
Difference Between pH and Buffer Compare the Difference Between Relationship Between Ph And Buffer Capacity Only the concentration of h + and. A buffer is a solution that resists a significant change. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. Β is buffer capacity (it is unitless) n is the number of moles. Relationship Between Ph And Buffer Capacity.
From www.researchgate.net
pH of distilled water and pH 7 buffer solution at various temperatures Relationship Between Ph And Buffer Capacity ph and buffers defined. A buffer is a solution that resists a significant change. Only the concentration of h + and. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. the buffer capacity is the amount of. Relationship Between Ph And Buffer Capacity.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Ph is a. Relationship Between Ph And Buffer Capacity.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts Relationship Between Ph And Buffer Capacity Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. therefore, to calculate buffer capacity, we use the following formula: the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity. Relationship Between Ph And Buffer Capacity.
From www.scribd.com
PH and Buffer Capacity PDF Ph Acid Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Only the concentration of h + and. Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. buffers • biological systems use buffers to. Relationship Between Ph And Buffer Capacity.
From www.youtube.com
pH and pKa relationship for buffers Chemistry Khan Academy YouTube Relationship Between Ph And Buffer Capacity A buffer is a solution that resists a significant change. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. ph and buffers defined. Ph is a measure of the concentration of h + [h 3 o +] ions. Relationship Between Ph And Buffer Capacity.
From www.researchgate.net
Buffer capacity versus pH of a seawater (SW) and pure water (DI Relationship Between Ph And Buffer Capacity ph and buffers defined. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. . Relationship Between Ph And Buffer Capacity.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Relationship Between Ph And Buffer Capacity Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added. Relationship Between Ph And Buffer Capacity.
From www.slideshare.net
Ph and buffer Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. the buffer. Relationship Between Ph And Buffer Capacity.
From www.researchgate.net
The effect of pH on the buffer capacity in wine. Download Scientific Relationship Between Ph And Buffer Capacity Only the concentration of h + and. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. buffers • biological systems use buffers to maintain ph. ph and buffers defined. therefore, to calculate buffer capacity, we use the following formula:. Relationship Between Ph And Buffer Capacity.
From www.scribd.com
pH and Buffers Acid Buffer Solution Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. buffers • biological systems use buffers to maintain ph. the buffer capacity is the amount of acid or base that can be added to a given volume of. Relationship Between Ph And Buffer Capacity.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. therefore, to calculate buffer capacity, we use the following formula: Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. ph and buffers. Relationship Between Ph And Buffer Capacity.
From www.slideserve.com
PPT General, Organic, and Biochemistry, 7e PowerPoint Presentation Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. therefore, to. Relationship Between Ph And Buffer Capacity.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Relationship Between Ph And Buffer Capacity Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that. Relationship Between Ph And Buffer Capacity.
From www.researchgate.net
Bulk buffer capacity (β) and bulk pH as a function of total buffer Relationship Between Ph And Buffer Capacity Only the concentration of h + and. A buffer is a solution that resists a significant change. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Ph is a measure of the concentration of h + [h 3 o +] ions in. Relationship Between Ph And Buffer Capacity.
From www.researchgate.net
Buffer capacity model specifications for a simulation interval between Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. A buffer is a solution that resists a significant change. Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. Only the. Relationship Between Ph And Buffer Capacity.
From www.youtube.com
Buffer capacity; making a buffer with a given pH value YouTube Relationship Between Ph And Buffer Capacity buffers • biological systems use buffers to maintain ph. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. Only the concentration of h + and. the buffer capacity is the amount of acid or base that can. Relationship Between Ph And Buffer Capacity.
From users.highland.edu
Buffers Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. therefore, to calculate buffer capacity, we use the following formula: Only the concentration of h + and. A buffer is a solution that resists a significant change. the. Relationship Between Ph And Buffer Capacity.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Only the concentration of h +. Relationship Between Ph And Buffer Capacity.
From www.researchgate.net
Relationship between pH and buffering capacity. Download Scientific Relationship Between Ph And Buffer Capacity Only the concentration of h + and. Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. ph and buffers defined. A buffer is a solution that resists a significant change. buffers • biological systems use buffers to maintain ph. the buffer capacity is the amount. Relationship Between Ph And Buffer Capacity.
From www.researchgate.net
Buffer capacity changes from initial pH to pH value 3 Download Relationship Between Ph And Buffer Capacity ph and buffers defined. Only the concentration of h + and. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. therefore, to calculate buffer capacity, we use the following formula: Β is buffer capacity (it is unitless). Relationship Between Ph And Buffer Capacity.
From www.reddit.com
Is it just me or does the bottom right graph contradict the Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. buffers • biological systems use buffers to maintain ph. therefore, to calculate buffer capacity, we use the following formula: the buffer capacity is the amount of acid or base that. Relationship Between Ph And Buffer Capacity.
From www.slideserve.com
PPT Chapter 13 Applications of Aqueous Equilibria PowerPoint Relationship Between Ph And Buffer Capacity A buffer is a solution that resists a significant change. Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. buffers • biological systems use buffers to maintain ph. ph and buffers defined. Only the concentration of h + and. the buffer capacity is the amount. Relationship Between Ph And Buffer Capacity.
From mungfali.com
PH And PKa Relationship Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. buffers • biological systems use buffers to maintain ph. Only the concentration of h + and. therefore, to calculate buffer capacity, we use the following formula: Ph is a measure of. Relationship Between Ph And Buffer Capacity.
From www.scribd.com
PH and Buffer System Notes PDF Buffer Solution Acid Relationship Between Ph And Buffer Capacity A buffer is a solution that resists a significant change. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. Only the. Relationship Between Ph And Buffer Capacity.
From chem-for-fun.blogspot.com
CHEMISTRY IS NOT HORRIBLE Buffers and Buffering Capacity Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. A buffer is. Relationship Between Ph And Buffer Capacity.
From www.youtube.com
Buffer Capacity and Choosing a Buffer based on pH YouTube Relationship Between Ph And Buffer Capacity A buffer is a solution that resists a significant change. Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. ph and buffers defined. Only the concentration of h + and. Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to. Relationship Between Ph And Buffer Capacity.
From www.toppr.com
The buffer capacity (beta) for a weak acid (A) conjugate base (B Relationship Between Ph And Buffer Capacity Only the concentration of h + and. Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. the buffer capacity is the amount of acid or base that can be. Relationship Between Ph And Buffer Capacity.
From www.slideserve.com
PPT Chapter 19 PowerPoint Presentation, free download ID4347161 Relationship Between Ph And Buffer Capacity Only the concentration of h + and. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. ph and buffers defined. Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. the buffer. Relationship Between Ph And Buffer Capacity.
From relationshipbetween.com
Difference Between Buffer Action And Buffer Capacity Relationship Between Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. A buffer is. Relationship Between Ph And Buffer Capacity.
From mmerevise.co.uk
pH Curves Questions and Revision MME Relationship Between Ph And Buffer Capacity buffers • biological systems use buffers to maintain ph. A buffer is a solution that resists a significant change. Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. the buffer capacity is the amount of acid or base that can be added to a given volume. Relationship Between Ph And Buffer Capacity.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Relationship Between Ph And Buffer Capacity Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added. Relationship Between Ph And Buffer Capacity.
From www.researchgate.net
Buffer capacity β H versus pH for twophase systems with Relationship Between Ph And Buffer Capacity Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. Only the concentration of h + and. A buffer is a solution that resists a significant change. therefore, to calculate. Relationship Between Ph And Buffer Capacity.
From saylordotorg.github.io
Buffers Relationship Between Ph And Buffer Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Β is buffer capacity (it is unitless) n is the number of moles of an acid or base (added to the. the buffer capacity is the amount of acid or base that. Relationship Between Ph And Buffer Capacity.
From pilgaard.info
Acids and bases Buffers Michael Pilgaard's Chemistry Relationship Between Ph And Buffer Capacity A buffer is a solution that resists a significant change. Ph is a measure of the concentration of h + [h 3 o +] ions in a solution. buffers • biological systems use buffers to maintain ph. therefore, to calculate buffer capacity, we use the following formula: the buffer capacity is the amount of acid or base. Relationship Between Ph And Buffer Capacity.