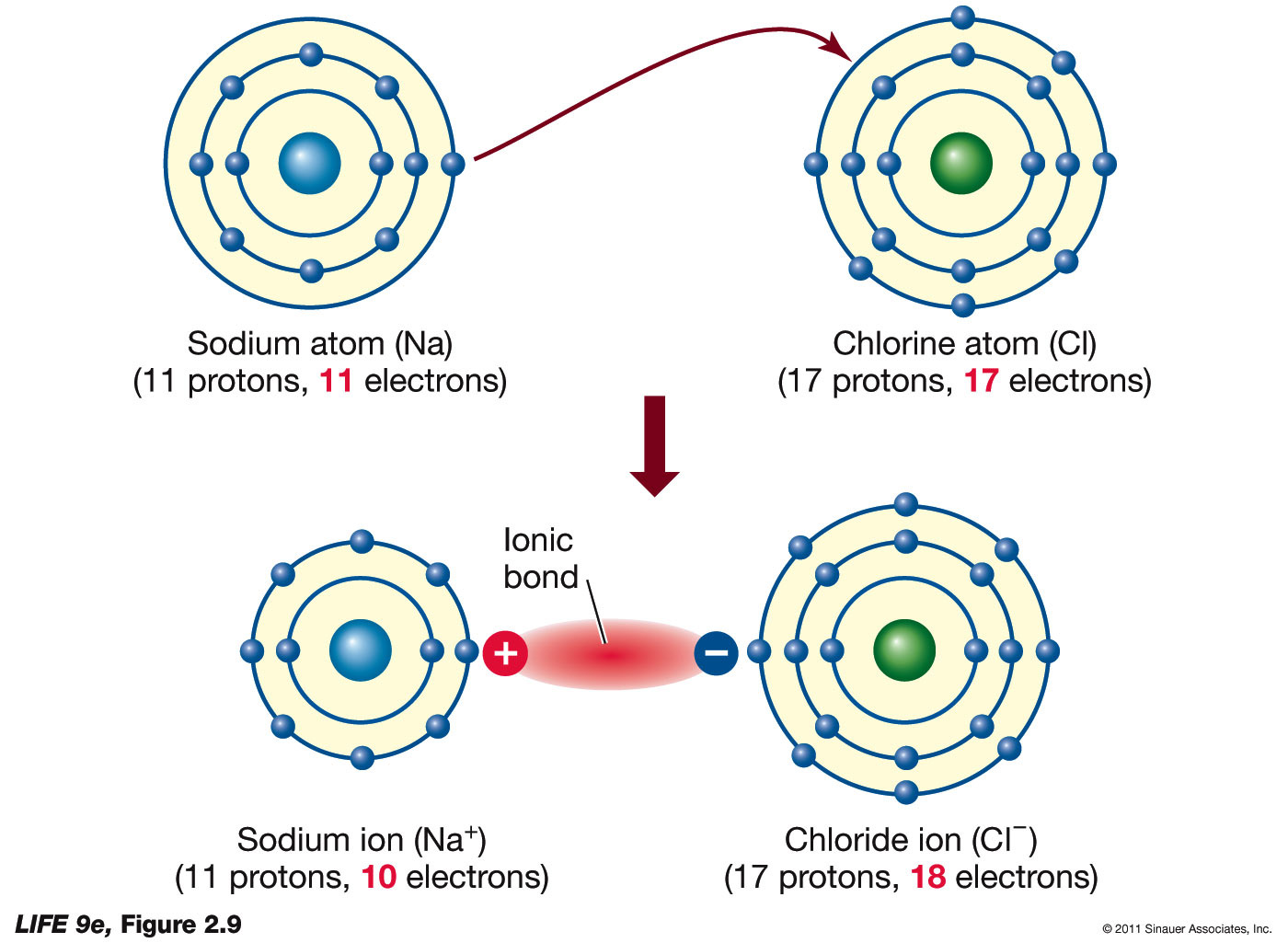
Ionic Bonds Metallic Elements . When the two ions combine via ionic bond, they form ionic compounds. These elements lie to the left in a. many metallic elements have relatively low ionization potentials and lose electrons easily. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. The atom that gains electrons becomes a negatively charged ion, known as an anion. These elements lie to the left in a. Learn more about ionic bonds in this article. the atom that loses electrons becomes a positively charged ion, known as a cation. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. many metallic elements have relatively low ionization potentials and lose electrons easily. alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties.
from derekcarrsavvy-chemist.blogspot.com
These elements lie to the left in a. When the two ions combine via ionic bond, they form ionic compounds. many metallic elements have relatively low ionization potentials and lose electrons easily. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Learn more about ionic bonds in this article. The atom that gains electrons becomes a negatively charged ion, known as an anion. learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. These elements lie to the left in a.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Ionic Bonds Metallic Elements These elements lie to the left in a. When the two ions combine via ionic bond, they form ionic compounds. The atom that gains electrons becomes a negatively charged ion, known as an anion. These elements lie to the left in a. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. many metallic elements have relatively low ionization potentials and lose electrons easily. learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Learn more about ionic bonds in this article. These elements lie to the left in a. the atom that loses electrons becomes a positively charged ion, known as a cation. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. many metallic elements have relatively low ionization potentials and lose electrons easily.
From www.tec-science.com
Metallic bonding tecscience Ionic Bonds Metallic Elements the atom that loses electrons becomes a positively charged ion, known as a cation. These elements lie to the left in a. learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. many metallic elements have relatively low ionization potentials and lose electrons easily. The atom that gains. Ionic Bonds Metallic Elements.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Periodicity (2) Melting and boiling points of the Ionic Bonds Metallic Elements Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. These elements lie to the left in a. the atom that loses electrons becomes a positively charged ion, known as a cation. When the two ions combine via ionic bond, they form ionic compounds. ionic bond, type of linkage formed. Ionic Bonds Metallic Elements.
From utedzz.blogspot.com
Periodic Table Ions List Periodic Table Timeline Ionic Bonds Metallic Elements ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. Learn more about ionic bonds in this article. These elements lie to the left in a. When the two ions. Ionic Bonds Metallic Elements.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Ionic Bonds Metallic Elements When the two ions combine via ionic bond, they form ionic compounds. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. many metallic elements have relatively low ionization potentials and lose electrons easily. many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie. Ionic Bonds Metallic Elements.
From www.chemistrylearner.com
Metallic Bond Definition, Examples, and Diagrams Ionic Bonds Metallic Elements alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.. Ionic Bonds Metallic Elements.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Ionic Bonds Metallic Elements many metallic elements have relatively low ionization potentials and lose electrons easily. learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. These elements lie to the left in a. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.. Ionic Bonds Metallic Elements.
From mungfali.com
Metallic Bonding Labelled Diagram Ionic Bonds Metallic Elements learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. These elements lie to the left in a. When the two ions combine via ionic bond, they form ionic compounds. The atom that gains electrons becomes a negatively charged ion, known as an anion. These elements lie to the left. Ionic Bonds Metallic Elements.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation ID5648526 Ionic Bonds Metallic Elements ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Learn more about ionic bonds in this article. When the two ions combine via ionic bond, they form ionic compounds. The atom that gains electrons becomes a negatively charged ion, known as an anion. learn about ionic and covalent bonding,. Ionic Bonds Metallic Elements.
From animalia-life.club
Metallic Bond Examples List Ionic Bonds Metallic Elements These elements lie to the left in a. The atom that gains electrons becomes a negatively charged ion, known as an anion. many metallic elements have relatively low ionization potentials and lose electrons easily. Learn more about ionic bonds in this article. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Ionic Bonds Metallic Elements.
From schematicdiagramyakuza.z13.web.core.windows.net
Diagram Of Metallic Bond Ionic Bonds Metallic Elements Learn more about ionic bonds in this article. These elements lie to the left in a. the atom that loses electrons becomes a positively charged ion, known as a cation. When the two ions combine via ionic bond, they form ionic compounds. These elements lie to the left in a. learn about ionic and covalent bonding, how metals. Ionic Bonds Metallic Elements.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Ionic Bonds Metallic Elements ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the atom that loses electrons becomes a positively charged ion, known as a cation. These elements lie to the left in a. These elements lie to the left in a. Such a bond forms when the valence (outermost) electrons of. Ionic Bonds Metallic Elements.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Ionic Bonds Metallic Elements When the two ions combine via ionic bond, they form ionic compounds. many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie to the left in a. These elements lie to the left in a. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.. Ionic Bonds Metallic Elements.
From www.slideshare.net
Chemical Structure Chemical Bonding. Ionic, Metallic & Coordinate Bo… Ionic Bonds Metallic Elements The atom that gains electrons becomes a negatively charged ion, known as an anion. Learn more about ionic bonds in this article. When the two ions combine via ionic bond, they form ionic compounds. many metallic elements have relatively low ionization potentials and lose electrons easily. learn about ionic and covalent bonding, how metals react to form ionic. Ionic Bonds Metallic Elements.
From learnwithdrscott.com
Ionic Bond Definition Easy Hard Science Ionic Bonds Metallic Elements Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. The atom that gains electrons becomes a negatively charged ion, known as an anion. When the two ions combine via ionic bond, they form ionic compounds. These elements lie to the left in a. the atom that loses electrons becomes a. Ionic Bonds Metallic Elements.
From www.dreamstime.com
Vector Illustration of a Metallic Bonding, Hydrogen Bonding,ionic Ionic Bonds Metallic Elements Learn more about ionic bonds in this article. learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. These elements lie to the left in a. many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie to the left in a. many metallic. Ionic Bonds Metallic Elements.
From en.wikipedia.org
Ionic bonding Wikipedia Ionic Bonds Metallic Elements learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. the atom that loses electrons becomes a positively charged ion, known as a cation. ionic bond, type. Ionic Bonds Metallic Elements.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Ionic Bonds Metallic Elements Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. many metallic elements have relatively low ionization potentials and lose electrons easily. alloys are commonly used in manufactured items because the. Ionic Bonds Metallic Elements.
From www.nuclear-power.com
Ionic Bond vs Metallic Bond Ionic Bonds Metallic Elements the atom that loses electrons becomes a positively charged ion, known as a cation. These elements lie to the left in a. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.. Ionic Bonds Metallic Elements.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Ionic Bonds Metallic Elements the atom that loses electrons becomes a positively charged ion, known as a cation. Learn more about ionic bonds in this article. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. These elements lie to the left in a. The atom that gains electrons becomes a negatively charged ion,. Ionic Bonds Metallic Elements.
From www.tes.com
Ionic, Covalent & Metallic Bonding (I)GCSE Chemistry Detailed Notes Ionic Bonds Metallic Elements many metallic elements have relatively low ionization potentials and lose electrons easily. alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. These elements lie to the left in a. These elements lie to the left in a. Learn more about ionic bonds in this article. Such a. Ionic Bonds Metallic Elements.
From stock.adobe.com
Isolated of chemical bonding on white background.vector illustration Ionic Bonds Metallic Elements These elements lie to the left in a. the atom that loses electrons becomes a positively charged ion, known as a cation. The atom that gains electrons becomes a negatively charged ion, known as an anion. Learn more about ionic bonds in this article. These elements lie to the left in a. learn about ionic and covalent bonding,. Ionic Bonds Metallic Elements.
From www.slideshare.net
Chemical Structure Chemical Bonding. Ionic, Metallic & Coordinate Bo… Ionic Bonds Metallic Elements ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. The atom that gains electrons becomes a negatively charged ion, known as an anion. When the two ions combine via ionic bond, they form ionic compounds. the atom that loses electrons becomes a positively charged ion, known as a cation.. Ionic Bonds Metallic Elements.
From scientifictutor.org
Chem Covalent, Ionic, and Metallic Bonds (Intramolecular Forces Ionic Bonds Metallic Elements many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie to the left in a. Learn more about ionic bonds in this article. alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. The atom that gains electrons becomes a negatively charged ion,. Ionic Bonds Metallic Elements.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Ionic Bonds Metallic Elements Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. These elements lie. Ionic Bonds Metallic Elements.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Ionic Bonds Metallic Elements alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. many metallic elements have relatively low ionization potentials and lose electrons easily. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. These elements lie to the left in. Ionic Bonds Metallic Elements.
From studymind.co.uk
Metallic Bonds (GCSE Chemistry) Study Mind Ionic Bonds Metallic Elements These elements lie to the left in a. learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. The atom that gains electrons becomes a negatively charged ion, known as an anion. These elements lie to the left in a. Such a bond forms when the valence (outermost) electrons of. Ionic Bonds Metallic Elements.
From www.slideserve.com
PPT Ionic and Metallic Bonding PowerPoint Presentation, free download Ionic Bonds Metallic Elements many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie to the left in a. These elements lie to the left in a. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. many metallic elements have relatively low ionization potentials and lose electrons easily.. Ionic Bonds Metallic Elements.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Ionic Bonds Metallic Elements alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the atom that loses electrons becomes a positively charged ion, known as a cation. These elements lie to the. Ionic Bonds Metallic Elements.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Ionic Bonds Metallic Elements When the two ions combine via ionic bond, they form ionic compounds. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie to the left in a. Such a bond forms when the valence (outermost). Ionic Bonds Metallic Elements.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Ionic Bonds Metallic Elements the atom that loses electrons becomes a positively charged ion, known as a cation. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. These elements lie to the. Ionic Bonds Metallic Elements.
From sciencenotes.org
Ionic Bond Definition and Examples Ionic Bonds Metallic Elements learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. These elements lie to the left in a. the atom that loses electrons becomes a positively charged ion, known as a cation. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another. Ionic Bonds Metallic Elements.
From www.chemistrylearner.com
Ionic Bond Facts, Definition, Properties, Examples, & Diagrams Ionic Bonds Metallic Elements The atom that gains electrons becomes a negatively charged ion, known as an anion. These elements lie to the left in a. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the atom that loses electrons becomes a positively charged ion, known as a cation. Learn more about ionic. Ionic Bonds Metallic Elements.
From www.science-revision.co.uk
Metallic bonding Ionic Bonds Metallic Elements many metallic elements have relatively low ionization potentials and lose electrons easily. the atom that loses electrons becomes a positively charged ion, known as a cation. Learn more about ionic bonds in this article. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. The atom that gains electrons becomes. Ionic Bonds Metallic Elements.
From mungfali.com
Metallic Bonding Labelled Diagram Ionic Bonds Metallic Elements many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie to the left in a. These elements lie to the left in a. Learn more about ionic bonds in this article. The atom that gains electrons becomes a negatively charged ion, known as an anion. Such a bond forms when the valence (outermost) electrons. Ionic Bonds Metallic Elements.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Ionic Bonds Metallic Elements Learn more about ionic bonds in this article. learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. many metallic elements have relatively low ionization potentials and lose electrons easily. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. . Ionic Bonds Metallic Elements.