Nitrogen Dioxide And Ammonia . ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. Implications for the nh 2 + no 2 reaction. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. the reaction of ammonia with nitrogen dioxide in a flow reactor:
from www.numerade.com
ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. the reaction of ammonia with nitrogen dioxide in a flow reactor: at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. Implications for the nh 2 + no 2 reaction.
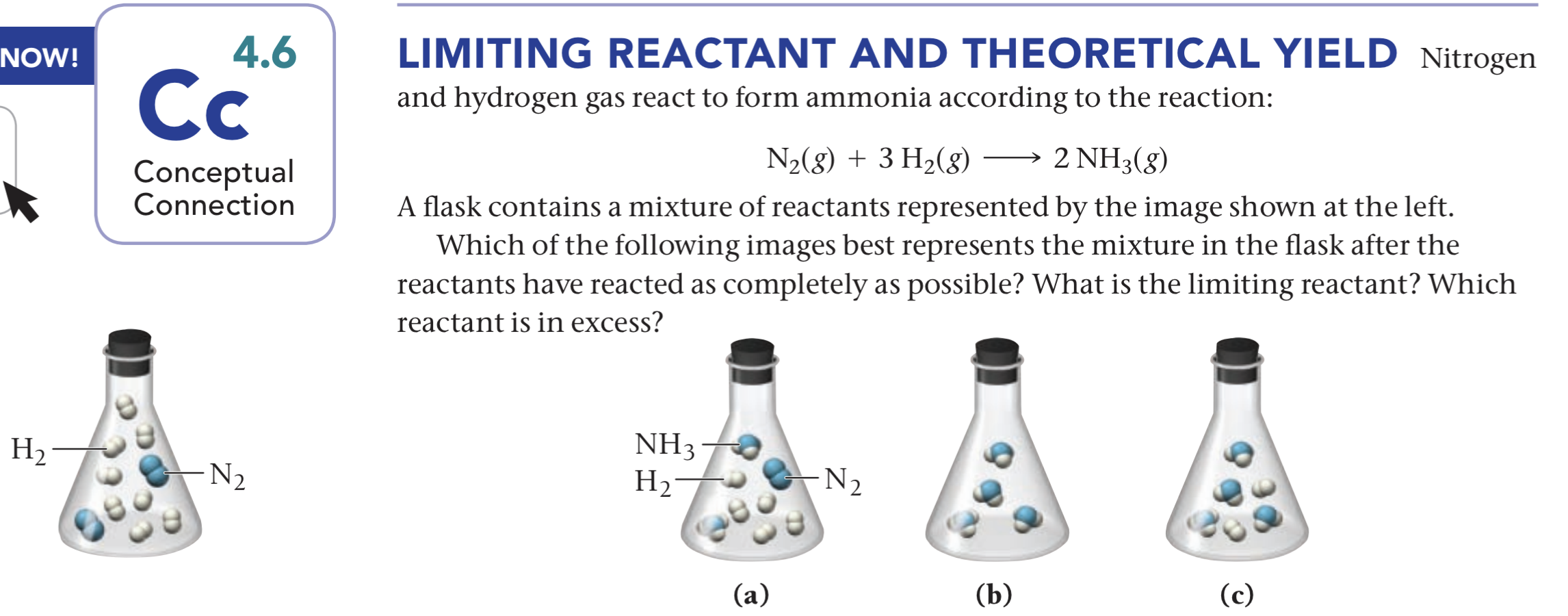
SOLVED Nitrogen and hydrogen gas react to form ammonia according to
Nitrogen Dioxide And Ammonia nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. the reaction of ammonia with nitrogen dioxide in a flow reactor: Implications for the nh 2 + no 2 reaction.
From www.researchgate.net
Nitrogen dioxide and ammonia sensing characteristics of graphene Nitrogen Dioxide And Ammonia ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no. Nitrogen Dioxide And Ammonia.
From www.numerade.com
SOLVED Nitrogen and hydrogen gas react to form ammonia according to Nitrogen Dioxide And Ammonia Implications for the nh 2 + no 2 reaction. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form.. Nitrogen Dioxide And Ammonia.
From www.poolspamarketing.com
Ammonia and nitrates in pools Understanding how these chemical Nitrogen Dioxide And Ammonia at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. Implications for the nh 2 + no 2 reaction. the reaction of ammonia with nitrogen dioxide in a flow reactor: nh3 + no2 = n2 + h2o is. Nitrogen Dioxide And Ammonia.
From www.pnas.org
Pathways of N2O production by marine ammoniaoxidizing archaea Nitrogen Dioxide And Ammonia ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. Implications for the nh 2 + no 2 reaction. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o. Nitrogen Dioxide And Ammonia.
From www.vedantu.com
Name the nitrifying bacteria of the soil. Why are they called Nitrogen Dioxide And Ammonia the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. Implications for the nh 2 + no 2 reaction. ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where. Nitrogen Dioxide And Ammonia.
From www.researchgate.net
Nitrogen dioxide and ammonia sensing characteristics of graphene Nitrogen Dioxide And Ammonia the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. nh3 + no2 = n2 + h2o is. Nitrogen Dioxide And Ammonia.
From www.pnas.org
Making ammonia from nitrogen and water microdroplets PNAS Nitrogen Dioxide And Ammonia Implications for the nh 2 + no 2 reaction. the reaction of ammonia with nitrogen dioxide in a flow reactor: the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form. Nitrogen Dioxide And Ammonia.
From testbook.com
Nitrogen Dioxide Structure, Valency, Formula, Properties, Effect Nitrogen Dioxide And Ammonia the reaction of ammonia with nitrogen dioxide in a flow reactor: the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. Implications for the nh 2 + no 2 reaction. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form. Nitrogen Dioxide And Ammonia.
From www.researchgate.net
(PDF) Nitrogen dioxide and ammonia gas molecules interaction studies on Nitrogen Dioxide And Ammonia ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. the reaction of ammonia with nitrogen dioxide in a flow reactor: the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. at elevated temperatures, n 2 reacts with h 2 to form. Nitrogen Dioxide And Ammonia.
From www.freepik.com
Premium Vector Structure of nitrogen dioxide molecule. no2 consisting Nitrogen Dioxide And Ammonia the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. Implications for the nh 2 + no 2 reaction. the reaction of ammonia with nitrogen dioxide in a flow reactor: ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. nh3 +. Nitrogen Dioxide And Ammonia.
From pubs.acs.org
Photocatalytic Conversion of Nitrogen to Ammonia with Water on Surface Nitrogen Dioxide And Ammonia at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. ammonia and nitrogen dioxide concentrations decrease. Nitrogen Dioxide And Ammonia.
From www.numerade.com
SOLVEDAmmonia reacts with oxygen to yield nitrogen and water. 4 NH3(g Nitrogen Dioxide And Ammonia at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. ammonia and nitrogen dioxide concentrations decrease monotonically with. Nitrogen Dioxide And Ammonia.
From www.science-revision.co.uk
Covalent bonding Nitrogen Dioxide And Ammonia at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. Implications for the nh 2 + no 2 reaction. the reaction of ammonia with nitrogen dioxide in a flow reactor: the low reactivity of ammonia (nh3) is the. Nitrogen Dioxide And Ammonia.
From www.earth.com
Why is the Nitrogen Cycle So Important? Nitrogen Dioxide And Ammonia nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. the reaction of ammonia with nitrogen dioxide in a flow reactor: Implications for the nh 2 + no 2 reaction. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as. Nitrogen Dioxide And Ammonia.
From www.globalseafood.org
Water quality standards Total Ammonia Nitrogen Responsible Seafood Nitrogen Dioxide And Ammonia the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. Implications for the nh 2 + no 2 reaction. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form.. Nitrogen Dioxide And Ammonia.
From www.gettyimages.com.au
35 Ammonia Molecule Stock Photos, HighRes Pictures, and Images Getty Nitrogen Dioxide And Ammonia nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. ammonia and nitrogen dioxide concentrations decrease. Nitrogen Dioxide And Ammonia.
From solvedlib.com
Ammonia is produced by the reaction of nitrogen and h… SolvedLib Nitrogen Dioxide And Ammonia at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. Implications for the nh 2 + no. Nitrogen Dioxide And Ammonia.
From www.numerade.com
SOLVED hydrogen gas combines with nitrogen to form ammonia Nitrogen Dioxide And Ammonia at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. ammonia and nitrogen dioxide concentrations decrease. Nitrogen Dioxide And Ammonia.
From www.dreamstime.com
Nitrogen Dioxide NO2 Balanced Reaction 3D Illustration Stock Nitrogen Dioxide And Ammonia at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. ammonia and nitrogen dioxide concentrations decrease monotonically with. Nitrogen Dioxide And Ammonia.
From www.bartleby.com
Answered Nitrogen monoxide and water react to… bartleby Nitrogen Dioxide And Ammonia nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2. Nitrogen Dioxide And Ammonia.
From heilprocessequipment.com
Nitrogen Oxide & Nitrogen Dioxide Heil Process Equipment Nitrogen Dioxide And Ammonia the reaction of ammonia with nitrogen dioxide in a flow reactor: at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of. Nitrogen Dioxide And Ammonia.
From www.coursehero.com
[Solved] Nitrogen monoxide and water react to form ammonia and oxygen Nitrogen Dioxide And Ammonia at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. the reaction of ammonia with nitrogen dioxide in a flow reactor: ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. Implications for the. Nitrogen Dioxide And Ammonia.
From www.chegg.com
Solved Nitrogen and hydrogen react to produce ammonia Nitrogen Dioxide And Ammonia the reaction of ammonia with nitrogen dioxide in a flow reactor: nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. at elevated temperatures,. Nitrogen Dioxide And Ammonia.
From www.studypool.com
SOLUTION Investigation of sensing mechanism of nasicon Nitrogen Dioxide And Ammonia Implications for the nh 2 + no 2 reaction. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon. Nitrogen Dioxide And Ammonia.
From www.bartleby.com
Answered Ammonia gas and oxygen gas react to… bartleby Nitrogen Dioxide And Ammonia Implications for the nh 2 + no 2 reaction. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form.. Nitrogen Dioxide And Ammonia.
From www.tessshebaylo.com
Equation For Ammonia Reacting With Water Tessshebaylo Nitrogen Dioxide And Ammonia ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. Implications for the nh 2 + no 2 reaction. the reaction of ammonia with nitrogen dioxide in a flow reactor: the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. at elevated. Nitrogen Dioxide And Ammonia.
From www.fraunhofer.de
The world’s first hightemperature ammoniapowered fuel cell for shipping Nitrogen Dioxide And Ammonia nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. Implications for the nh 2 + no 2 reaction. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. at elevated temperatures, n 2 reacts. Nitrogen Dioxide And Ammonia.
From stock.adobe.com
Elements and Compounds are compared in the molecular structure. Oxygen Nitrogen Dioxide And Ammonia the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. Implications for the nh 2 + no 2 reaction. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where. Nitrogen Dioxide And Ammonia.
From www.studypool.com
SOLUTION Investigation of sensing mechanism of nasicon Nitrogen Dioxide And Ammonia Implications for the nh 2 + no 2 reaction. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o. Nitrogen Dioxide And Ammonia.
From www.orangeth.com
Nitrogen Dioxide NO2 พิษภัย Nitrogen Dioxide And Ammonia ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. Implications for the nh 2 + no 2 reaction. the low reactivity of ammonia. Nitrogen Dioxide And Ammonia.
From www.numerade.com
SOLVED Ammonia burns in air (with a platinum catalyst), producing Nitrogen Dioxide And Ammonia ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. Implications for the nh 2 + no 2 reaction. the reaction of ammonia with. Nitrogen Dioxide And Ammonia.
From www.numerade.com
SOLVED Urea (H₂NCONH₂) is used extensively as a nitrogen source in Nitrogen Dioxide And Ammonia Implications for the nh 2 + no 2 reaction. the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where. Nitrogen Dioxide And Ammonia.
From brainly.in
Translate the following statements into chemical equations and then Nitrogen Dioxide And Ammonia Implications for the nh 2 + no 2 reaction. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. the reaction of ammonia with nitrogen dioxide in a flow reactor: ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. . Nitrogen Dioxide And Ammonia.
From www.alamy.com
Molecular Model of Nitrogen Dioxide (NO2) Molecule. Vector Illustration Nitrogen Dioxide And Ammonia the low reactivity of ammonia (nh3) is the main barrier to applying neat nh3 as fuel in technical applications, such. nh3 + no2 = n2 + h2o is a double displacement (metathesis) reaction where eight moles of ammonia [nh 3] and six. ammonia and nitrogen dioxide concentrations decrease monotonically with increasing temperature, with the no 2. Implications. Nitrogen Dioxide And Ammonia.
From www.numerade.com
SOLVED Ammonia reacts with oxygen to produce nitrogen dioxide and Nitrogen Dioxide And Ammonia at elevated temperatures, n 2 reacts with h 2 to form ammonia, with o 2 to form a mixture of no and no 2, and with carbon to form. the reaction of ammonia with nitrogen dioxide in a flow reactor: Implications for the nh 2 + no 2 reaction. ammonia and nitrogen dioxide concentrations decrease monotonically with. Nitrogen Dioxide And Ammonia.