What Is Necessary For A Molecule To Be Considered Polar . a bond between two or more atoms is polar if the atoms have significantly different electronegativities (>0.4). because of the shape of the molecule and the polar —oh grouping in methanol, we expect its molecules to be polar and for it. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. Contain at least one polar covalent bond. Polar bonds do not share electrons equally,. to be considered a polar bond, the difference in electronegativity must be large. like bonds, molecules can also be polar. to summarize, to be polar, a molecule must: a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. Have a molecular structure such that the sum of the vectors of each. The dipole moment points in the direction of the.
from ar.inspiredpencil.com
Polar bonds do not share electrons equally,. to summarize, to be polar, a molecule must: a bond between two or more atoms is polar if the atoms have significantly different electronegativities (>0.4). because of the shape of the molecule and the polar —oh grouping in methanol, we expect its molecules to be polar and for it. Have a molecular structure such that the sum of the vectors of each. The dipole moment points in the direction of the. like bonds, molecules can also be polar. understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. to be considered a polar bond, the difference in electronegativity must be large. Contain at least one polar covalent bond.
Polar Molecule Definition
What Is Necessary For A Molecule To Be Considered Polar a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. because of the shape of the molecule and the polar —oh grouping in methanol, we expect its molecules to be polar and for it. Polar bonds do not share electrons equally,. to summarize, to be polar, a molecule must: Have a molecular structure such that the sum of the vectors of each. Contain at least one polar covalent bond. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. The dipole moment points in the direction of the. a bond between two or more atoms is polar if the atoms have significantly different electronegativities (>0.4). to be considered a polar bond, the difference in electronegativity must be large. a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. like bonds, molecules can also be polar. understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting.
From www.britannica.com
polarity Definition & Examples Britannica What Is Necessary For A Molecule To Be Considered Polar to summarize, to be polar, a molecule must: a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. because of the shape of the molecule and the polar —oh grouping in methanol, we expect its molecules to be polar and. What Is Necessary For A Molecule To Be Considered Polar.
From ar.inspiredpencil.com
Polar Molecule Definition What Is Necessary For A Molecule To Be Considered Polar a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. to be considered a polar bond, the difference in electronegativity must. What Is Necessary For A Molecule To Be Considered Polar.
From stilleducation.com
Polar and Nonpolar Covalent Bonds Characteristics & Differences What Is Necessary For A Molecule To Be Considered Polar understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. to summarize, to be polar, a molecule must: Have a molecular structure such that the sum of the vectors of. What Is Necessary For A Molecule To Be Considered Polar.
From tutor-t.blogspot.com
How To Know If A Molecule Is Polar Covalent What Is Necessary For A Molecule To Be Considered Polar The dipole moment points in the direction of the. like bonds, molecules can also be polar. because of the shape of the molecule and the polar —oh grouping in methanol, we expect its molecules to be polar and for it. a bond between two or more atoms is polar if the atoms have significantly different electronegativities (>0.4).. What Is Necessary For A Molecule To Be Considered Polar.
From www.slideserve.com
PPT Polar or Nonpolar PowerPoint Presentation, free download ID3483667 What Is Necessary For A Molecule To Be Considered Polar In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. like bonds, molecules can also be polar. a bond between two or more atoms is polar if the atoms have significantly different electronegativities (>0.4). The dipole moment points in the direction of the. because of the shape. What Is Necessary For A Molecule To Be Considered Polar.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts What Is Necessary For A Molecule To Be Considered Polar The dipole moment points in the direction of the. a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. Have a molecular structure such that the sum of the vectors of each. understanding the polarity of a molecule is essential in. What Is Necessary For A Molecule To Be Considered Polar.
From printableabrirme8x.z22.web.core.windows.net
Polarity Of Molecules And Its Properties Ppt What Is Necessary For A Molecule To Be Considered Polar to be considered a polar bond, the difference in electronegativity must be large. to summarize, to be polar, a molecule must: a bond between two or more atoms is polar if the atoms have significantly different electronegativities (>0.4). like bonds, molecules can also be polar. Contain at least one polar covalent bond. Have a molecular structure. What Is Necessary For A Molecule To Be Considered Polar.
From ar.inspiredpencil.com
Polar Molecule Definition What Is Necessary For A Molecule To Be Considered Polar In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. The dipole moment points in the direction of the. like bonds, molecules can also be polar. Polar bonds do not share electrons equally,. to summarize, to be polar, a molecule must: a bond between two or more. What Is Necessary For A Molecule To Be Considered Polar.
From charlesbasomann.blogspot.com
What is a Polar Molecule What Is Necessary For A Molecule To Be Considered Polar Have a molecular structure such that the sum of the vectors of each. because of the shape of the molecule and the polar —oh grouping in methanol, we expect its molecules to be polar and for it. understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. like bonds,. What Is Necessary For A Molecule To Be Considered Polar.
From www.slideserve.com
PPT ELECTRONEGATIVITY POLAR BONDS MOLECULAR POLARITY PowerPoint What Is Necessary For A Molecule To Be Considered Polar to be considered a polar bond, the difference in electronegativity must be large. Have a molecular structure such that the sum of the vectors of each. The dipole moment points in the direction of the. understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. like bonds, molecules can. What Is Necessary For A Molecule To Be Considered Polar.
From biologydictionary.net
Polar Molecule Definition and Examples Biology Dictionary What Is Necessary For A Molecule To Be Considered Polar a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. to summarize, to be polar, a molecule must: understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. Polar bonds do not. What Is Necessary For A Molecule To Be Considered Polar.
From ar.inspiredpencil.com
Polar Molecule Definition What Is Necessary For A Molecule To Be Considered Polar Contain at least one polar covalent bond. because of the shape of the molecule and the polar —oh grouping in methanol, we expect its molecules to be polar and for it. Polar bonds do not share electrons equally,. understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. like. What Is Necessary For A Molecule To Be Considered Polar.
From pediaa.com
Difference Between Polar and Nonpolar Molecules Definition, Formation What Is Necessary For A Molecule To Be Considered Polar In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. to summarize, to be polar, a molecule must: like bonds, molecules can also be polar. Contain at least one polar covalent bond. understanding the polarity of a molecule is essential in chemistry, as it affects various properties,. What Is Necessary For A Molecule To Be Considered Polar.
From www.youtube.com
Polar Molecules Tutorial How to determine polarity in a molecule YouTube What Is Necessary For A Molecule To Be Considered Polar In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. Have a molecular structure such that the sum of the vectors of each. like bonds, molecules can also be polar. to be considered a polar bond, the difference in electronegativity must be large. a bond between two. What Is Necessary For A Molecule To Be Considered Polar.
From www.chegg.com
Solved What is necessary for a molecule to be considered What Is Necessary For A Molecule To Be Considered Polar understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. Contain at least one polar covalent bond. Polar bonds do not share electrons equally,. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. because of the shape of the. What Is Necessary For A Molecule To Be Considered Polar.
From guidealegrasejv.z13.web.core.windows.net
How To Identify A Polar Covalent Bond What Is Necessary For A Molecule To Be Considered Polar to summarize, to be polar, a molecule must: Have a molecular structure such that the sum of the vectors of each. like bonds, molecules can also be polar. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. Contain at least one polar covalent bond. a bond. What Is Necessary For A Molecule To Be Considered Polar.
From www.youtube.com
Molecule Polarity Chemistry Tutorial YouTube What Is Necessary For A Molecule To Be Considered Polar Contain at least one polar covalent bond. to be considered a polar bond, the difference in electronegativity must be large. Have a molecular structure such that the sum of the vectors of each. The dipole moment points in the direction of the. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial. What Is Necessary For A Molecule To Be Considered Polar.
From tutor-t.blogspot.com
How To Know If A Molecule Is Polar Covalent What Is Necessary For A Molecule To Be Considered Polar to summarize, to be polar, a molecule must: In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. Contain at least one polar covalent bond. like bonds, molecules can also be polar. a bond between two or more atoms is polar if the atoms have significantly different. What Is Necessary For A Molecule To Be Considered Polar.
From nl.pinterest.com
Why Is Water a Polar Molecule? Hydrogen Atom, Hydrogen Bond, Ionic What Is Necessary For A Molecule To Be Considered Polar a bond between two or more atoms is polar if the atoms have significantly different electronegativities (>0.4). a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. Polar bonds do not share electrons equally,. like bonds, molecules can also be. What Is Necessary For A Molecule To Be Considered Polar.
From sciencenotes.org
Polar and Nonpolar Molecules What Is Necessary For A Molecule To Be Considered Polar The dipole moment points in the direction of the. Have a molecular structure such that the sum of the vectors of each. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. Contain at least one polar covalent bond. to be considered a polar bond, the difference in electronegativity. What Is Necessary For A Molecule To Be Considered Polar.
From www.slideserve.com
PPT CHEMICAL BONDS PowerPoint Presentation, free download ID5623361 What Is Necessary For A Molecule To Be Considered Polar Polar bonds do not share electrons equally,. to be considered a polar bond, the difference in electronegativity must be large. Have a molecular structure such that the sum of the vectors of each. understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. because of the shape of the. What Is Necessary For A Molecule To Be Considered Polar.
From www.pinterest.com
molecular polarity table Molecular geometry, Molecular shapes, Molecular What Is Necessary For A Molecule To Be Considered Polar a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. Polar bonds do not share electrons equally,. understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. to be considered a polar. What Is Necessary For A Molecule To Be Considered Polar.
From socratic.org
What kinds of molecules are polar? + Example What Is Necessary For A Molecule To Be Considered Polar In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. because of the shape of the molecule and the polar —oh. What Is Necessary For A Molecule To Be Considered Polar.
From www.slideshare.net
Molecular polarity What Is Necessary For A Molecule To Be Considered Polar because of the shape of the molecule and the polar —oh grouping in methanol, we expect its molecules to be polar and for it. like bonds, molecules can also be polar. Polar bonds do not share electrons equally,. to be considered a polar bond, the difference in electronegativity must be large. In a polar molecule, electron density. What Is Necessary For A Molecule To Be Considered Polar.
From www.youtube.com
Polar and Nonpolar Molecules YouTube What Is Necessary For A Molecule To Be Considered Polar Have a molecular structure such that the sum of the vectors of each. to be considered a polar bond, the difference in electronegativity must be large. a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. a bond between two. What Is Necessary For A Molecule To Be Considered Polar.
From www.electricity-magnetism.org
¿Qué es un momento dipolar eléctrico? What Is Necessary For A Molecule To Be Considered Polar to summarize, to be polar, a molecule must: like bonds, molecules can also be polar. Polar bonds do not share electrons equally,. to be considered a polar bond, the difference in electronegativity must be large. a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the. What Is Necessary For A Molecule To Be Considered Polar.
From www.chegg.com
Solved Q2. Benzoic acid is considered to be a polar What Is Necessary For A Molecule To Be Considered Polar Have a molecular structure such that the sum of the vectors of each. The dipole moment points in the direction of the. to summarize, to be polar, a molecule must: a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. In. What Is Necessary For A Molecule To Be Considered Polar.
From mavink.com
Polar Molecules Lewis Structure What Is Necessary For A Molecule To Be Considered Polar a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. like bonds, molecules can also be polar. Have a molecular structure such that the sum of the vectors of each. Contain at least one polar covalent bond. Polar bonds do not. What Is Necessary For A Molecule To Be Considered Polar.
From www.slideserve.com
PPT Chapter 8 Molecular Geometry and Polarity PowerPoint What Is Necessary For A Molecule To Be Considered Polar understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. to be considered a polar bond, the difference in electronegativity must be large. like bonds, molecules can also be polar. a bond between two or more atoms is polar if the atoms have significantly different electronegativities (>0.4). Have. What Is Necessary For A Molecule To Be Considered Polar.
From bazaabigaillyman.blogspot.com
What is a Polar Molecule Abigail Lyman What Is Necessary For A Molecule To Be Considered Polar Have a molecular structure such that the sum of the vectors of each. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. understanding the polarity of a molecule is essential in chemistry, as it affects various properties, including solubility, melting. Polar bonds do not share electrons equally,. . What Is Necessary For A Molecule To Be Considered Polar.
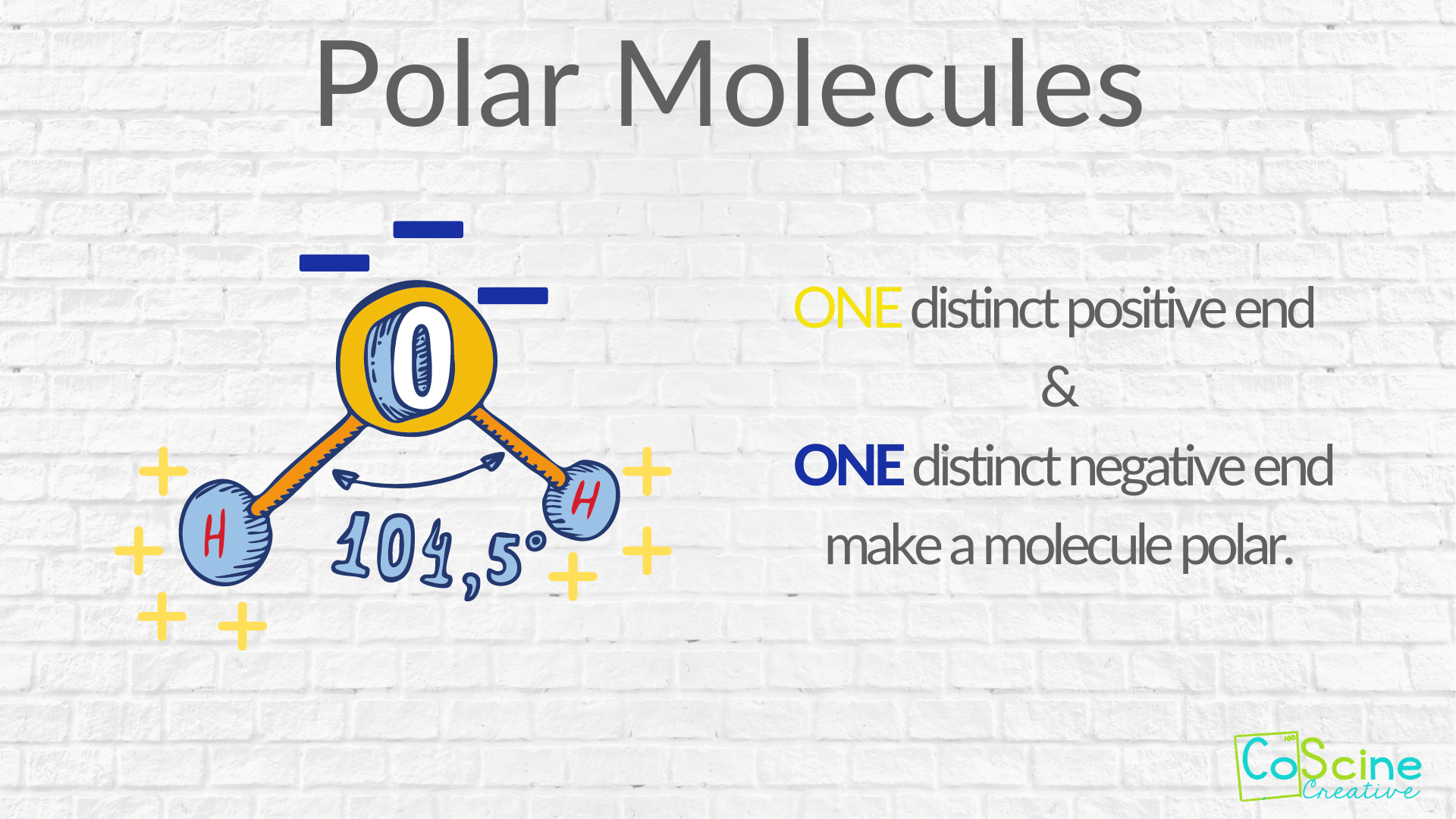
From www.coscinecreative.com
How Do You Teach Polar Vs. Nonpolar Molecules? — CoScine Creative What Is Necessary For A Molecule To Be Considered Polar to summarize, to be polar, a molecule must: a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. Have a molecular structure such that the sum of the vectors of each. because of the shape of the molecule and the. What Is Necessary For A Molecule To Be Considered Polar.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii What Is Necessary For A Molecule To Be Considered Polar In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. Have a molecular structure such that the sum of the vectors of each. The dipole moment points in the direction of the. Contain at least one polar covalent bond. a molecule is polar if it has a dipole, where. What Is Necessary For A Molecule To Be Considered Polar.
From www.slideshare.net
2012 Molecule Polarity What Is Necessary For A Molecule To Be Considered Polar to be considered a polar bond, the difference in electronegativity must be large. because of the shape of the molecule and the polar —oh grouping in methanol, we expect its molecules to be polar and for it. a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and. What Is Necessary For A Molecule To Be Considered Polar.
From www.chegg.com
Solved Which of the following would be considered a polar What Is Necessary For A Molecule To Be Considered Polar to be considered a polar bond, the difference in electronegativity must be large. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. Have a molecular structure such that the sum of the vectors of each. understanding the polarity of a molecule is essential in chemistry, as it. What Is Necessary For A Molecule To Be Considered Polar.
From www.thoughtco.com
Why Is Water a Polar Molecule? What Is Necessary For A Molecule To Be Considered Polar a molecule is polar if it has a dipole, where one side of the molecule has a positive charge, and the other side has a negative charge. to be considered a polar bond, the difference in electronegativity must be large. to summarize, to be polar, a molecule must: like bonds, molecules can also be polar. . What Is Necessary For A Molecule To Be Considered Polar.