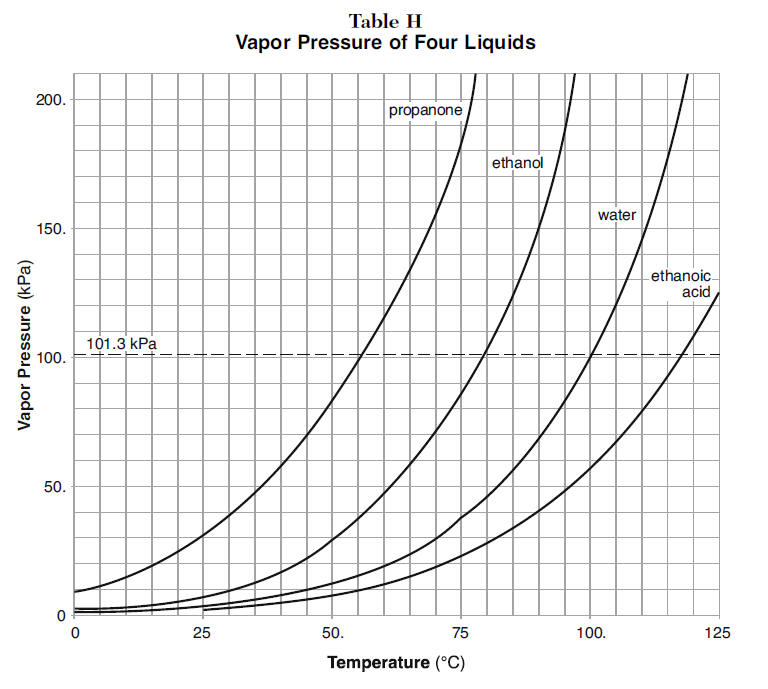
What Is Vapor Pressure Boiling . Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Boiling is the rapid vaporization of. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of.
from mr.kentchemistry.com
The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Boiling is the rapid vaporization of. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of.
Determine Boiling Point from Vapor Pressure
What Is Vapor Pressure Boiling Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Boiling is the rapid vaporization of. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization.
From www.slideserve.com
PPT Chapter 11 Gases PowerPoint Presentation, free download ID1586364 What Is Vapor Pressure Boiling Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Boiling is the. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download What Is Vapor Pressure Boiling Boiling is the rapid vaporization of. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. The. What Is Vapor Pressure Boiling.
From mr.kentchemistry.com
Determine Boiling Point from Vapor Pressure What Is Vapor Pressure Boiling Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. Boiling is the rapid vaporization of. The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. The vapor pressure of a liquid is the. What Is Vapor Pressure Boiling.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is Vapor Pressure Boiling The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. The main difference between vapor. What Is Vapor Pressure Boiling.
From mungfali.com
Vapor Pressure And Boiling Point Relationship What Is Vapor Pressure Boiling That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. Boiling is the rapid vaporization of. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with. What Is Vapor Pressure Boiling.
From saylordotorg.github.io
Vapor Pressure What Is Vapor Pressure Boiling Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Boiling is the. What Is Vapor Pressure Boiling.
From www.worksheetsplanet.com
What is Boiling Point What Is Vapor Pressure Boiling The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. Boiling. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Energy & Changes of State PowerPoint Presentation ID What Is Vapor Pressure Boiling The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. That is, the pressure of the vapor resulting from evaporation. What Is Vapor Pressure Boiling.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is Vapor Pressure Boiling The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Boiling is the rapid vaporization of.. What Is Vapor Pressure Boiling.
From socratic.org
What is the relation between critical temperature and boiling point or What Is Vapor Pressure Boiling The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is. What Is Vapor Pressure Boiling.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts What Is Vapor Pressure Boiling The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. Vapor pressure is the pressure. What Is Vapor Pressure Boiling.
From morioh.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation What Is Vapor Pressure Boiling The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along. What Is Vapor Pressure Boiling.
From www.youtube.com
Vapour Pressure and Boiling Point Relationship Vapour Pressure vs What Is Vapor Pressure Boiling Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Boiling is the rapid vaporization of. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The normal. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Aim What is the relationship between vapor pressure and boiling What Is Vapor Pressure Boiling The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Boiling. What Is Vapor Pressure Boiling.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel What Is Vapor Pressure Boiling Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The normal boiling. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Is Vapor Pressure Boiling Boiling is the rapid vaporization of. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is the pressure exerted by any liquid over its surface at a particular. What Is Vapor Pressure Boiling.
From www.youtube.com
Vapor Pressure & Boiling Point YouTube What Is Vapor Pressure Boiling Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is the pressure exerted by any. What Is Vapor Pressure Boiling.
From mavink.com
Vapor Pressure And Boiling Point Relationship What Is Vapor Pressure Boiling The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is the pressure exerted by a. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download What Is Vapor Pressure Boiling The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. That. What Is Vapor Pressure Boiling.
From www.youtube.com
Which Compound Has a Higher Vapor Pressure? Intermolecular Force What Is Vapor Pressure Boiling That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. The. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download What Is Vapor Pressure Boiling The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Boiling is the rapid vaporization of. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with. What Is Vapor Pressure Boiling.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapor Pressure Boiling The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. That is, the. What Is Vapor Pressure Boiling.
From study.com
Vapor Pressure Definition, Equation & Examples Video & Lesson What Is Vapor Pressure Boiling Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The main difference between vapor pressure and boiling point is. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free What Is Vapor Pressure Boiling Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Explore more on liquid state vapour pressure, nature. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Air Pressure, Vapor Pressure, & Boiling PowerPoint Presentation What Is Vapor Pressure Boiling Boiling is the rapid vaporization of. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. That is, the pressure of the vapor resulting from evaporation of. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Air Pressure, Vapor Pressure, & Boiling PowerPoint Presentation What Is Vapor Pressure Boiling The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. Boiling is the rapid vaporization of. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at. What Is Vapor Pressure Boiling.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Is Vapor Pressure Boiling Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. Boiling is the rapid vaporization of. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure Boiling Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. Boiling is the rapid vaporization. What Is Vapor Pressure Boiling.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Is Vapor Pressure Boiling The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Boiling. What Is Vapor Pressure Boiling.
From www.researchgate.net
The vapor pressure of ethanol vs. the normal boilingpoint temperature What Is Vapor Pressure Boiling Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. That is, the pressure of. What Is Vapor Pressure Boiling.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube What Is Vapor Pressure Boiling The main difference between vapor pressure and boiling point is that vapor pressure is a measurement of pressure whereas boiling point is a measurement of. Boiling is the rapid vaporization of. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. The normal boiling point is the temperature in. What Is Vapor Pressure Boiling.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is Vapor Pressure Boiling Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. Explore more on liquid state vapour pressure, nature of the liquids, effect of temperature, boiling point along with heat of vaporization. Vapor pressure is the pressure. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT The Gaseous State of Matter PowerPoint Presentation, free What Is Vapor Pressure Boiling The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to. Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 What Is Vapor Pressure Boiling Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The main difference between vapor pressure and boiling. What Is Vapor Pressure Boiling.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 What Is Vapor Pressure Boiling Vapor pressure is the pressure exerted by any liquid over its surface at a particular temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Explore more on liquid state vapour pressure, nature of the. What Is Vapor Pressure Boiling.