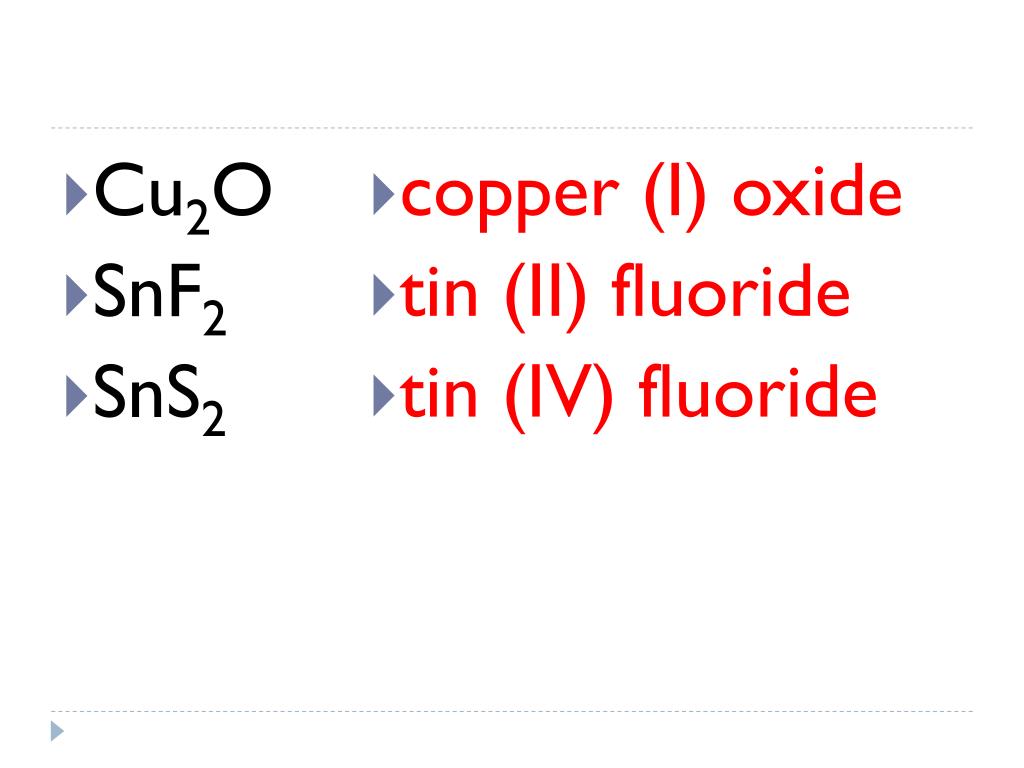
Tin (Iv) Oxide Cation . It has tin and oxide ions in it. In the solid state, ionic. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. Ionic compounds do not exist as molecules. Recognize polyatomic ions in chemical formulas. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. If you're seeing this message, it means we're having trouble loading external resources on our website. The mineral form of sno 2 is called cassiterite , and. All the anions are of this type, gaining the number of electrons. Its chemical formula is sno 2. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound.
from www.slideserve.com
It has tin and oxide ions in it. In the solid state, ionic. If you're seeing this message, it means we're having trouble loading external resources on our website. All the anions are of this type, gaining the number of electrons. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. Its chemical formula is sno 2. Recognize polyatomic ions in chemical formulas. The mineral form of sno 2 is called cassiterite , and. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states.
PPT Naming Ions PowerPoint Presentation, free download ID4897123
Tin (Iv) Oxide Cation Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. It has tin and oxide ions in it. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. In the solid state, ionic. All the anions are of this type, gaining the number of electrons. Its chemical formula is sno 2. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. The mineral form of sno 2 is called cassiterite , and. If you're seeing this message, it means we're having trouble loading external resources on our website. Recognize polyatomic ions in chemical formulas. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. Ionic compounds do not exist as molecules.
From www.numerade.com
SOLVED Ionic Compounds with Variable Charges Write the Symbol and Tin (Iv) Oxide Cation Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. The mineral form of sno 2 is called cassiterite , and. Its chemical formula is sno 2. In the solid state, ionic. All the anions are of this type, gaining the number of electrons. It has tin and oxide ions in it. Tin(iv) oxide, also known as. Tin (Iv) Oxide Cation.
From www.fishersci.no
Potassium tin(IV) oxide trihydrate, 95+ (metals basis), Thermo Tin (Iv) Oxide Cation Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. In the solid state, ionic. The mineral form of sno 2 is called cassiterite , and. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. Recognize polyatomic ions in chemical formulas. Its chemical formula is sno 2. All. Tin (Iv) Oxide Cation.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID2696147 Tin (Iv) Oxide Cation All the anions are of this type, gaining the number of electrons. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Its chemical formula is sno 2. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. The mineral form of sno 2 is called cassiterite , and. It has. Tin (Iv) Oxide Cation.
From www.numerade.com
SOLVED For the equation SnO2 + 2H2 Sn + 2H2O, tin (IV) oxide reacts Tin (Iv) Oxide Cation The mineral form of sno 2 is called cassiterite , and. It has tin and oxide ions in it. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. Ionic compounds do not exist as molecules. All the anions are of this type, gaining the number of electrons. Tin (iv) oxide, also known as. Tin (Iv) Oxide Cation.
From www.flinnsci.com
Ion Names, Formulas and Charges Chart Flinn Scientific Tin (Iv) Oxide Cation Its chemical formula is sno 2. The mineral form of sno 2 is called cassiterite , and. In the solid state, ionic. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Ionic compounds do not exist as molecules. Recognize polyatomic ions in chemical formulas. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with. Tin (Iv) Oxide Cation.
From slideplayer.com
Chemistry NOMENCLATURE ppt download Tin (Iv) Oxide Cation If you're seeing this message, it means we're having trouble loading external resources on our website. In the solid state, ionic. Its chemical formula is sno 2. The mineral form of sno 2 is called cassiterite , and. It has tin and oxide ions in it. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Tin(iv). Tin (Iv) Oxide Cation.
From www.researchgate.net
Electrical conductivity of the synthesized tin (IV) oxide samples Tin (Iv) Oxide Cation In the solid state, ionic. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. All the anions are of this type, gaining the number of. Tin (Iv) Oxide Cation.
From www.pv-magazine.com
Triple cation perovskite PV cell with ETL based on tin(IV) oxide hits Tin (Iv) Oxide Cation Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. In the solid state, ionic. Ionic compounds do not exist as molecules. Its chemical formula is sno 2. The mineral form of sno 2 is called cassiterite , and. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Recognize polyatomic ions. Tin (Iv) Oxide Cation.
From www.researchgate.net
SEM images of tin (IV) oxide (a) and sulfated tin (IV) oxide (b Tin (Iv) Oxide Cation All the anions are of this type, gaining the number of electrons. Ionic compounds do not exist as molecules. Recognize polyatomic ions in chemical formulas. The mineral form of sno 2 is called cassiterite , and. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. It has tin and oxide ions in it.. Tin (Iv) Oxide Cation.
From www.semanticscholar.org
Figure 1 from Precursors for the Formation of Tin(IV) Oxide and Related Tin (Iv) Oxide Cation Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. It has tin and oxide ions in it. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. Recognize polyatomic ions in chemical formulas. All the anions are of this type, gaining the number of electrons. Ionic compounds do. Tin (Iv) Oxide Cation.
From www.numerade.com
SOLVED 1. Tin, Sn, commonly forms two cations tin(II), Sn2+; and tin Tin (Iv) Oxide Cation Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. In the solid state, ionic. Ionic compounds do not exist as molecules. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. If you're seeing this message, it means we're having trouble loading external resources on our website. Its chemical formula is. Tin (Iv) Oxide Cation.
From www.slideserve.com
PPT 1 st Semester Exam in High School Chemistry PowerPoint Tin (Iv) Oxide Cation Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. The mineral form of sno 2 is called cassiterite , and. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. Its chemical formula is sno 2. Recognize polyatomic ions in chemical formulas. All the anions are of this. Tin (Iv) Oxide Cation.
From sciencekitstore.com
Tin Oxide, Stannic Oxide, Tin (IV) Oxide, SnO2, 99.8 Tin (Iv) Oxide Cation The mineral form of sno 2 is called cassiterite , and. It has tin and oxide ions in it. Its chemical formula is sno 2. If you're seeing this message, it means we're having trouble loading external resources on our website. In the solid state, ionic. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula. Tin (Iv) Oxide Cation.
From butchixanh.edu.vn
Top 7+ tin iv sulfide formula Latest Bút Chì Xanh Tin (Iv) Oxide Cation Recognize polyatomic ions in chemical formulas. In the solid state, ionic. If you're seeing this message, it means we're having trouble loading external resources on our website. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. The mineral. Tin (Iv) Oxide Cation.
From www.slideshare.net
Ionic bonding binary Tin (Iv) Oxide Cation Recognize polyatomic ions in chemical formulas. The mineral form of sno 2 is called cassiterite , and. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. It has tin and oxide ions in it. All the anions are of this type, gaining the number of electrons. If you're seeing this message, it means we're. Tin (Iv) Oxide Cation.
From slideplayer.com
Chapter 3 Lecture Outline ppt download Tin (Iv) Oxide Cation Its chemical formula is sno 2. All the anions are of this type, gaining the number of electrons. In the solid state, ionic. Recognize polyatomic ions in chemical formulas. It has tin and oxide ions in it. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. Figure 2.7.2 lists the ions (cation and. Tin (Iv) Oxide Cation.
From www.shutterstock.com
Tin Iv Oxide Images Browse 4 Stock Photos & Vectors Free Download with Tin (Iv) Oxide Cation Ionic compounds do not exist as molecules. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Recognize polyatomic ions in chemical formulas. If you're seeing this message, it means we're having trouble loading external resources on our website. Its chemical formula is sno 2. The mineral form of sno 2 is called cassiterite , and. All. Tin (Iv) Oxide Cation.
From www.researchgate.net
XRD spectrum pattern of tin (IV) oxide and sulfated tin (IV) oxide (a Tin (Iv) Oxide Cation If you're seeing this message, it means we're having trouble loading external resources on our website. The mineral form of sno 2 is called cassiterite , and. It has tin and oxide ions in it. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. Ionic compounds do not exist as molecules. Recognize polyatomic ions. Tin (Iv) Oxide Cation.
From www.slideserve.com
PPT I. Ion Formation Ionic Formulas Ionic Nomenclature PowerPoint Tin (Iv) Oxide Cation Recognize polyatomic ions in chemical formulas. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Its chemical formula is sno 2. If you're seeing this message, it means we're having trouble loading external resources on our website. It has tin. Tin (Iv) Oxide Cation.
From chemcraft.su
Tin(IV) oxide, 99.9 pure p.a. chemcraft.su Tin (Iv) Oxide Cation It has tin and oxide ions in it. Its chemical formula is sno 2. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. If you're seeing this message, it means we're having trouble loading external resources on our website. In the solid state, ionic. The mineral form of sno 2 is called cassiterite , and. Tin(iv). Tin (Iv) Oxide Cation.
From www.cell.com
Epoxidation of Argemone mexicana oil with peroxyacetic acid formed in Tin (Iv) Oxide Cation Its chemical formula is sno 2. It has tin and oxide ions in it. The mineral form of sno 2 is called cassiterite , and. In the solid state, ionic. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this type, gaining the number of electrons. Tin (iv) oxide, also known. Tin (Iv) Oxide Cation.
From www.chegg.com
Solved Consider the reaction of tin(IV) oxide with carbon. Tin (Iv) Oxide Cation The mineral form of sno 2 is called cassiterite , and. Recognize polyatomic ions in chemical formulas. It has tin and oxide ions in it. In the solid state, ionic. If you're seeing this message, it means we're having trouble loading external resources on our website. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Ionic. Tin (Iv) Oxide Cation.
From www.youtube.com
How to write the formula for titanium (IV) oxide YouTube Tin (Iv) Oxide Cation All the anions are of this type, gaining the number of electrons. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Recognize polyatomic ions in chemical formulas. If you're seeing this message, it means we're having trouble loading external resources on our website. Ionic compounds do not exist as molecules. The mineral form of sno 2. Tin (Iv) Oxide Cation.
From www.youtube.com
How to Write the Formula for Tin (IV) oxide YouTube Tin (Iv) Oxide Cation It has tin and oxide ions in it. Recognize polyatomic ions in chemical formulas. All the anions are of this type, gaining the number of electrons. In the solid state, ionic. Ionic compounds do not exist as molecules. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. Tin(iv) oxide, also known as stannic oxide,. Tin (Iv) Oxide Cation.
From www.youtube.com
How to write chemical formula Tin IV Oxide YouTube Tin (Iv) Oxide Cation Recognize polyatomic ions in chemical formulas. Its chemical formula is sno 2. Ionic compounds do not exist as molecules. In the solid state, ionic. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. All the anions are of this type, gaining the number of electrons. The mineral form of sno 2 is called cassiterite. Tin (Iv) Oxide Cation.
From www.chegg.com
Solved Write the balanced chemical equation for the Tin (Iv) Oxide Cation Ionic compounds do not exist as molecules. In the solid state, ionic. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. The mineral form of sno 2 is called cassiterite , and. Its chemical formula is sno 2. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. Tin (iv). Tin (Iv) Oxide Cation.
From www.researchgate.net
SEM images of tin (IV) oxide (a) and sulfated tin (IV) oxide (b Tin (Iv) Oxide Cation Its chemical formula is sno 2. Recognize polyatomic ions in chemical formulas. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. In the solid state, ionic. All the anions are of this type, gaining the number of electrons. It has tin and oxide ions in it. Tin (iv) oxide, also known as tin dioxide or stannic. Tin (Iv) Oxide Cation.
From particularmaterials.com
Tin (IV) Oxide, SnO2 nanoparticles Particular Materials SRL Tin (Iv) Oxide Cation Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. In the solid state, ionic. It has tin and oxide ions in it. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Recognize polyatomic ions in chemical formulas. If you're seeing this message, it means we're having trouble loading external. Tin (Iv) Oxide Cation.
From www.numerade.com
SOLVED Fill in the names and formulas for ions and compounds as Tin (Iv) Oxide Cation Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Its chemical formula is sno 2. The mineral form of sno 2 is called cassiterite , and. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno. Tin (Iv) Oxide Cation.
From www.slideserve.com
PPT Chemical Names and Formulas PowerPoint Presentation, free Tin (Iv) Oxide Cation Its chemical formula is sno 2. Recognize polyatomic ions in chemical formulas. If you're seeing this message, it means we're having trouble loading external resources on our website. All the anions are of this type, gaining the number of electrons. It has tin and oxide ions in it. The mineral form of sno 2 is called cassiterite , and. Tin(iv). Tin (Iv) Oxide Cation.
From www.americanelements.com
Tin(IV) Oxide AMERICAN ELEMENTS Tin (Iv) Oxide Cation In the solid state, ionic. Ionic compounds do not exist as molecules. It has tin and oxide ions in it. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. If you're seeing this message, it means we're having trouble loading external resources on our website. Tin (iv) oxide, also known as tin dioxide or stannic oxide. Tin (Iv) Oxide Cation.
From www.fishersci.com
Tin(IV) oxide, 15 in H{2}O colloidal dispersion, Thermo Scientific Tin (Iv) Oxide Cation Recognize polyatomic ions in chemical formulas. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. Its chemical formula is sno 2. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. If you're seeing this message, it means we're having trouble loading external resources on our website. In. Tin (Iv) Oxide Cation.
From www.researchgate.net
Ligandstabilized cations of germanium, tin and lead in oxidation state Tin (Iv) Oxide Cation If you're seeing this message, it means we're having trouble loading external resources on our website. It has tin and oxide ions in it. All the anions are of this type, gaining the number of electrons. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. The mineral form of sno 2 is called cassiterite , and.. Tin (Iv) Oxide Cation.
From www.slideserve.com
PPT Naming Ions PowerPoint Presentation, free download ID4897123 Tin (Iv) Oxide Cation Ionic compounds do not exist as molecules. All the anions are of this type, gaining the number of electrons. Recognize polyatomic ions in chemical formulas. Tin (iv) oxide, also known as tin dioxide or stannic oxide is a chemical compound. Tin(iv) oxide, also known as stannic oxide, is the inorganic compound with the formula sno 2. In the solid state,. Tin (Iv) Oxide Cation.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496 Tin (Iv) Oxide Cation If you're seeing this message, it means we're having trouble loading external resources on our website. It has tin and oxide ions in it. Ionic compounds do not exist as molecules. Its chemical formula is sno 2. Recognize polyatomic ions in chemical formulas. The mineral form of sno 2 is called cassiterite , and. Tin(iv) oxide, also known as stannic. Tin (Iv) Oxide Cation.