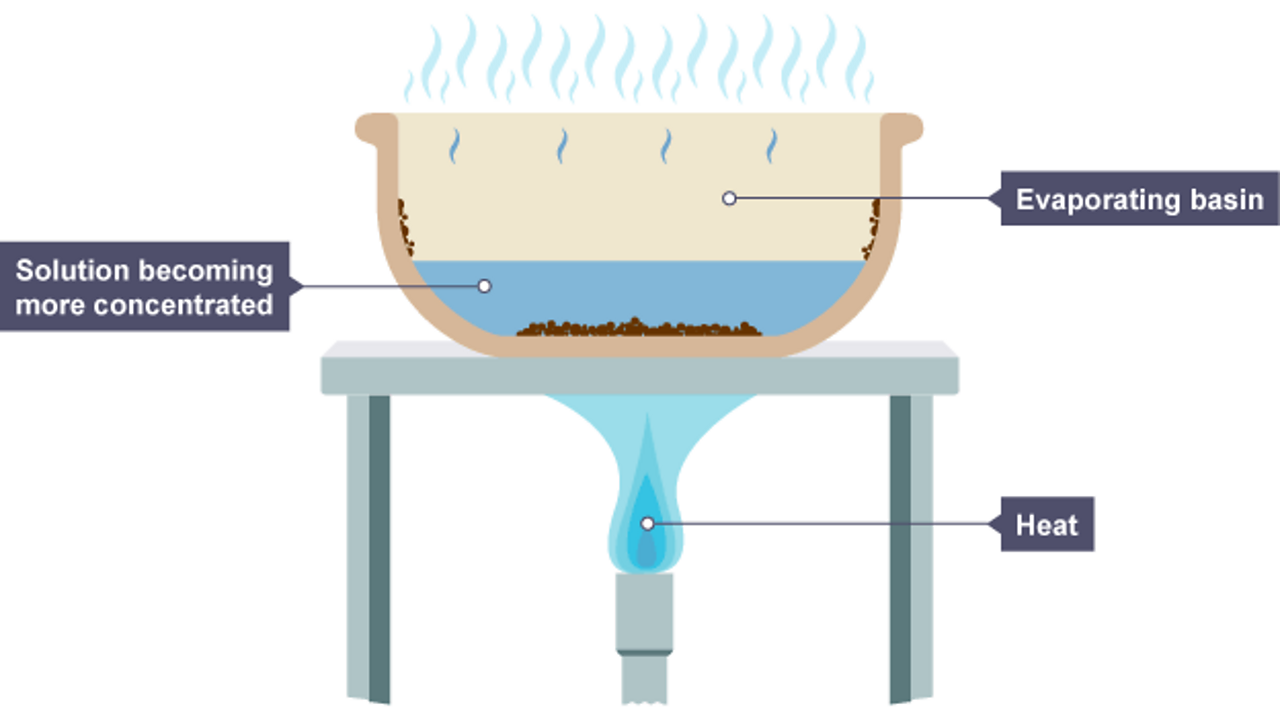
What Is The Evaporation Method . Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. Evaporation is great for separating a mixture. Evaporation causes the cooling exchange of energy. The change from liquid phase to gas phase is called. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy.
from www.bbc.co.uk
Evaporation is great for separating a mixture. The change from liquid phase to gas phase is called. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation causes the cooling exchange of energy. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state.
Evaporation BBC Bitesize
What Is The Evaporation Method Evaporation causes the cooling exchange of energy. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation causes the cooling exchange of energy. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. Evaporation is great for separating a mixture. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. The change from liquid phase to gas phase is called.
From www.slideserve.com
PPT EVAPORATION PowerPoint Presentation, free download ID311632 What Is The Evaporation Method Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. The change from liquid phase to gas phase is called. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. Crystallisation is a. What Is The Evaporation Method.
From www.teachoo.com
11+ Examples of Evaporation in our daily life (Explained!) Teachoo What Is The Evaporation Method Evaporation causes the cooling exchange of energy. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation is great for separating a mixture. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation occurs when a liquid slowly turns. What Is The Evaporation Method.
From www.youtube.com
What is evaporation How salt is made Evaporation process & facts What Is The Evaporation Method Evaporation is great for separating a mixture. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation causes the cooling exchange of energy. Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas. What Is The Evaporation Method.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii What Is The Evaporation Method To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. Crystallisation is a separation technique used to obtain. What Is The Evaporation Method.
From colosoimages.com
evaporation . labeled liquid to gas state process scheme Coloso What Is The Evaporation Method Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation is great for separating a mixture. Evaporation. What Is The Evaporation Method.
From ar.inspiredpencil.com
Evaporation Chemistry What Is The Evaporation Method To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation causes the cooling exchange of energy. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. Evaporation,. What Is The Evaporation Method.
From www.slideserve.com
PPT SEPARATION TECHNIQUES PowerPoint Presentation, free download ID What Is The Evaporation Method The change from liquid phase to gas phase is called. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation causes the cooling exchange of energy. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation is great for separating a mixture. To. What Is The Evaporation Method.
From study.com
Evaporation Definition, Process & Examples Lesson What Is The Evaporation Method Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. Crystallisation is a separation technique used to obtain crystals of a solid solute. The change from liquid phase to gas phase is called. Evaporation is great for separating a mixture. To isolate the solute,. What Is The Evaporation Method.
From www.researchgate.net
Solvent evaporation method of preparation for nanoparticles Download What Is The Evaporation Method Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation is great for separating a mixture. The change from liquid phase to gas phase is called. Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. Evaporation. What Is The Evaporation Method.
From saltworkconsultants.com
Physics of evaporation What Is The Evaporation Method To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is great for separating a mixture. Evaporation is the process in which a liquid changes to the gaseous state. What Is The Evaporation Method.
From www.narodnatribuna.info
Evaporation Separating Mixtures What Is The Evaporation Method Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. The change from liquid phase to gas phase is called. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation is the process in which a liquid changes to the gaseous state as the free. What Is The Evaporation Method.
From jayceegrozhang.blogspot.com
Evaporation Is the Process Where a Liquid Changes From Its What Is The Evaporation Method Evaporation is great for separating a mixture. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. To isolate the solute, it would. What Is The Evaporation Method.
From galliumstem.com
Water Cycle Gallium STEM What Is The Evaporation Method Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. Crystallisation is a separation technique used to obtain crystals of a solid solute. The change from liquid phase to gas phase is called. To isolate the solute, it would be a simple matter of waiting while the solvent molecules. What Is The Evaporation Method.
From www.youtube.com
Class 6 Science Separation of Substances Evaporation and Condensation What Is The Evaporation Method Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation is great for separating a mixture. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. The change from liquid phase to gas phase is called. Evaporation causes the cooling exchange of energy. To isolate. What Is The Evaporation Method.
From www.vedantu.com
Evaporation Learn Important Terms and Concepts What Is The Evaporation Method Evaporation causes the cooling exchange of energy. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is great for separating a mixture. Evaporation, process by which an element. What Is The Evaporation Method.
From www.aplustopper.com
How can we Separate a Mixture of a Solid and a Liquid using Evaporation What Is The Evaporation Method Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation causes the cooling exchange of energy. Evaporation is great for separating a mixture. Evaporation occurs when a liquid slowly turns into a gas below its. What Is The Evaporation Method.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo What Is The Evaporation Method Crystallisation is a separation technique used to obtain crystals of a solid solute. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation causes the cooling exchange of energy. The change from liquid phase to gas phase is called. Evaporation is the process. What Is The Evaporation Method.
From www.slideserve.com
PPT GEO3020/4020 Lecture 2 I. Energy balance II. Evapotranspiration What Is The Evaporation Method Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas. What Is The Evaporation Method.
From www.slideserve.com
PPT Separating Mixtures PowerPoint Presentation, free download ID What Is The Evaporation Method Crystallisation is a separation technique used to obtain crystals of a solid solute. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation is great for separating a mixture. Evaporation is the process in which a liquid changes to the gaseous state as. What Is The Evaporation Method.
From edu.rsc.org
Evaporation explained Infographic RSC Education What Is The Evaporation Method The change from liquid phase to gas phase is called. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the. What Is The Evaporation Method.
From mavink.com
Labelled Diagram Of Evaporation What Is The Evaporation Method Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation causes the cooling exchange of energy. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from. What Is The Evaporation Method.
From www.youtube.com
Separation of Substances Class 6 Science Chapter 5 Evaporation and What Is The Evaporation Method Crystallisation is a separation technique used to obtain crystals of a solid solute. The change from liquid phase to gas phase is called. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. Evaporation is the process by which water (and other liquids) changes. What Is The Evaporation Method.
From www.yaclass.in
Evaporation — lesson. Science CBSE, Class 9. What Is The Evaporation Method Crystallisation is a separation technique used to obtain crystals of a solid solute. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation causes the cooling exchange of energy. The change from liquid phase to gas phase is called. Evaporation is great for. What Is The Evaporation Method.
From collegedunia.com
Factor Affecting Evaporation Definition, Rate of Evaporation, Examples What Is The Evaporation Method Evaporation occurs when a liquid slowly turns into a gas below its boiling point. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. What Is The Evaporation Method.
From www.geeksforgeeks.org
Separation by Evaporation What Is The Evaporation Method Evaporation is great for separating a mixture. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. The. What Is The Evaporation Method.
From www.slideserve.com
PPT Evaporation PowerPoint Presentation, free download ID2598333 What Is The Evaporation Method Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation causes the cooling exchange of energy. Evaporation is the process in which a liquid changes to the. What Is The Evaporation Method.
From www.researchgate.net
Preparation of NPs by emulsion evaporation method. Download What Is The Evaporation Method Evaporation is great for separating a mixture. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation causes the cooling exchange of energy. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. The change from liquid phase to. What Is The Evaporation Method.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo What Is The Evaporation Method Evaporation is great for separating a mixture. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation causes the cooling exchange of. What Is The Evaporation Method.
From mungfali.com
Evaporation Technique What Is The Evaporation Method Evaporation is great for separating a mixture. Evaporation causes the cooling exchange of energy. Evaporation is the process by which water (and other liquids) changes from a liquid state to a vapor or gas state. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Crystallisation is a separation technique used to obtain crystals of a. What Is The Evaporation Method.
From www.teachoo.com
Why is Evaporation called a surface phenomenon? Teachoo What Is The Evaporation Method Evaporation causes the cooling exchange of energy. The change from liquid phase to gas phase is called. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation is the process in which a liquid changes. What Is The Evaporation Method.
From www.teachoo.com
11+ Examples of Evaporation in our daily life (Explained!) Teachoo What Is The Evaporation Method Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation is great for separating a mixture. Evaporation causes the cooling exchange of energy. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation, process by which an element or. What Is The Evaporation Method.
From www.vecteezy.com
Diagram showing Evaporation Separating Mixtures 3176406 Vector Art at What Is The Evaporation Method Crystallisation is a separation technique used to obtain crystals of a solid solute. The change from liquid phase to gas phase is called. Evaporation is the process in which a liquid changes to the gaseous state as the free surface, below its boiling point, through the transfer of energy. To isolate the solute, it would be a simple matter of. What Is The Evaporation Method.
From www.bigditch.com.au
Evaporation management 101 Big Ditch Dam Company What Is The Evaporation Method Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. The change from liquid phase to gas phase is called. Evaporation is. What Is The Evaporation Method.
From www.bbc.co.uk
Evaporation BBC Bitesize What Is The Evaporation Method Evaporation is great for separating a mixture. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. To isolate the solute, it. What Is The Evaporation Method.
From civilmint.com
What is Evaporation, its process, & methods of estimation What Is The Evaporation Method To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. The change from liquid phase to gas phase is called. Evaporation causes the cooling exchange of energy. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation is the process. What Is The Evaporation Method.