What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture . Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. We have to calculate the volume of water before we dilution the question. The initial concentration is 25%, the final concentration is. 20 people found it helpful. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. I think it is equal because if 20 ml sugar and 50 ml. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. The sugar adds about 9/16 of. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are.
from www.chegg.com
When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. I think it is equal because if 20 ml sugar and 50 ml. We have to calculate the volume of water before we dilution the question. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. The initial concentration is 25%, the final concentration is. The sugar adds about 9/16 of. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. 20 people found it helpful.
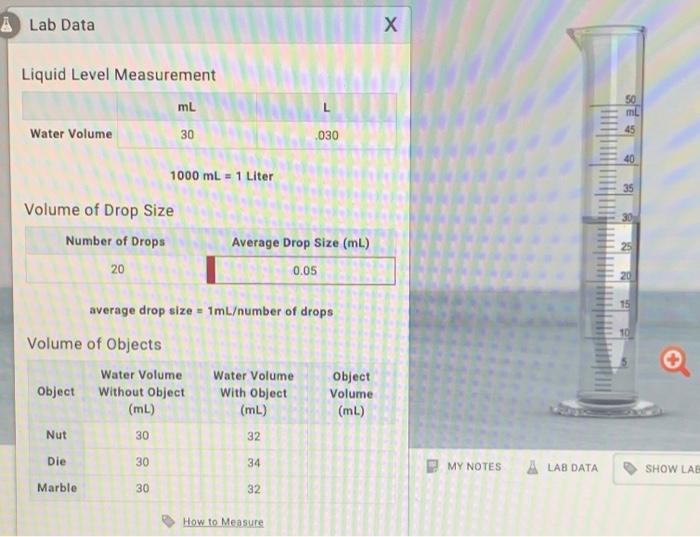
Solved Lab Data Х Liquid Level Measurement mL L 50 mi Water
What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture The initial concentration is 25%, the final concentration is. I think it is equal because if 20 ml sugar and 50 ml. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. The initial concentration is 25%, the final concentration is. We have to calculate the volume of water before we dilution the question. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. The sugar adds about 9/16 of. 20 people found it helpful.
From www.chegg.com
Solved Lab Data Х Liquid Level Measurement mL L 50 mi Water What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture The sugar adds about 9/16 of. The initial concentration is 25%, the final concentration is. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. 20 people found it helpful. We have to calculate the volume of water before we dilution the question. Is the volume of the. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.coursehero.com
[Solved] answer pls. 2. (1 pt) Determine the volume of water associated What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture I think it is equal because if 20 ml sugar and 50 ml. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. The initial concentration is 25%, the final concentration is. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.toppr.com
A solution of glucose (molar mass = 180 g mol^1 ) in water is labelled What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture The initial concentration is 25%, the final concentration is. We have to calculate the volume of water before we dilution the question. I think it is equal because if 20 ml sugar and 50 ml. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. The sugar adds. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From brainly.in
Calculate The Molarity of 0.840M of Sugar (C12H22O11) Solution (Density What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. Is the volume of the resulting sugar mixture equal, more than or less than the sum (20. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.toppr.com
2.5 A solution of glucose in water is labelled as 10 w/w, what would What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. I think it is equal because if 20 ml sugar and 50 ml. The initial concentration is 25%, the final concentration is. We have to calculate the volume of water before we dilution the question. Is the volume. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From brainly.com
The diagram below represents the creation of a sugar solution by adding What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. The sugar adds about 9/16 of. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. Is the volume. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.youtube.com
Calculate the mass percentage composition of Glucose (C6H12O6). YouTube What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. The initial concentration is 25%, the final concentration is. I think it is equal because if 20. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.chegg.com
Solved [5 Points] 4. With reference to the solubility of What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture We have to calculate the volume of water before we dilution the question. The sugar adds about 9/16 of. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. 20 people found it helpful. Is the volume of the resulting sugar mixture equal, more than or less than the. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. We have to calculate the volume of water before we dilution the question. The initial concentration is 25%, the final concentration is. I think it is equal because if 20. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.chegg.com
Liquid Level Measurement mL L Water Volume 30 0.03 What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture 20 people found it helpful. We have to calculate the volume of water before we dilution the question. I think it is equal because if 20 ml sugar and 50 ml. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. The sugar adds about 9/16 of. When sugar. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.sweetashoney.co
How Many ml In A Tablespoon Sweet As Honey What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture I think it is equal because if 20 ml sugar and 50 ml. The sugar adds about 9/16 of. The initial concentration is 25%, the final concentration is. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. We have to calculate the volume of water before we dilution. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.pipeen.com
1 Bottle Of Water Equals How Many Liters Best Pictures and Decription What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. The initial. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.chegg.com
Solved 1) A solution is prepared by dissolving 28.4 g of What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. The initial concentration is 25%, the final concentration is. 20 people found it helpful. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From summeryule.com
12 oz in ml (Convert 12 oz to ml) • Summer Yule Nutrition and Recipes What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture 20 people found it helpful. I think it is equal because if 20 ml sugar and 50 ml. The initial concentration is 25%, the final concentration is. Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. The sugar adds about 9/16. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.numerade.com
SOLVEDA solution of glucose in water is labelled as 10 w / w, what What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. The initial concentration is 25%, the final concentration is. 20 people found it helpful. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.nagwa.com
Question Video Using Measuring Jugs to Compare Volume in Milliliters What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture The sugar adds about 9/16 of. We have to calculate the volume of water before we dilution the question. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. 20 people found it helpful. Is the volume of the resulting sugar mixture equal to, more than, or less than. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From byjus.com
The volume of water that a submerged object displaces is equal to the What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. 20 people found it helpful. We have to calculate the volume of water before we dilution the question. The initial concentration is 25%, the final concentration is. I think it is equal. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From brainly.in
A solution contains 'x' ml of ethyl alcohol mixed with 100 ml of water What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture 20 people found it helpful. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. However, when mixing. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From askfilo.com
6. A solution of glucose (molar mass =180 g mol−1 ) in water is labelled What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture 20 people found it helpful. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. I think it is. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From app.emaze.com
Mixtures and solutions on emaze What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. 20 people found it helpful. We have to. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.numerade.com
12.5g of glucose (C6H12O6) is dissolved in enough water to make 750.0mL What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. We have to calculate the volume of water before we dilution the question. I think it is equal because if 20 ml sugar and 50 ml. The initial concentration is 25%, the final concentration is. Is the volume of. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.toppr.com
How many grams of glucose C_6H_{12}O_6 should be dissolved in 0.5 kg of What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture 20 people found it helpful. I think it is equal because if 20 ml sugar and 50 ml. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From summeryule.com
8 oz to ml (Convert 8 ounces to ml) • Summer Yule Nutrition and Recipes What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture I think it is equal because if 20 ml sugar and 50 ml. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.slideserve.com
PPT Natural Approach to Chemistry Chapter 9 Water & Solutions What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture 20 people found it helpful. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. When sugar is dissolved in water, the sugar molecules fit into the spaces between the water molecules, resulting in a decrease in. We have to. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From byjus.com
Estimate the volume of water in the second beaker. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. We have to calculate the volume of water before we dilution the question. 20 people found it helpful. Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.youtube.com
A solution of glucose in water is labelled as 10 w/w, what would be What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. However, when. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From byjus.com
A solution of glucose in water is labelled as 10 What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture 20 people found it helpful. The initial concentration is 25%, the final concentration is. The sugar adds about 9/16 of. Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. When sugar is dissolved in water, the sugar molecules fit into the. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture The initial concentration is 25%, the final concentration is. I think it is equal because if 20 ml sugar and 50 ml. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. When sugar is dissolved in water, the sugar. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.numerade.com
SOLVED A student placed 11.0 g of glucose (C6H12O6) in a volumetric What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture We have to calculate the volume of water before we dilution the question. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. The initial concentration is 25%, the final concentration is. 20 people found it helpful. I think it. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.numerade.com
SOLVEDDetermine the density (g / mL) for each of the following a. A What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture The sugar adds about 9/16 of. 20 people found it helpful. I think it is equal because if 20 ml sugar and 50 ml. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. The initial concentration is 25%, the final concentration is. When sugar is dissolved in water,. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.toppr.com
34.2 g of glucose is dissolved in 400 g of water. Calculate percentage What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture The initial concentration is 25%, the final concentration is. I think it is equal because if 20 ml sugar and 50 ml. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. 20 people found it helpful. We have to calculate the volume of water before we dilution the. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.numerade.com
SOLVED A student placed 19.0 g of glucose C6H12O6 in a volumetric What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture The initial concentration is 25%, the final concentration is. Is the volume of the resulting sugar mixture equal to, more than, or less than the sum of the volumes of 20ml of sugar and 50ml of water, which are. The sugar adds about 9/16 of. I think it is equal because if 20 ml sugar and 50 ml. However, when. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.numerade.com
SOLVEDCalculate the volume in milliliters of a 1.420 M NaOH solution What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. Is the volume of the resulting sugar mixture equal, more than or less than the sum (20 ml sugar +50ml water ) of the volumes of the unmixed. 20 people found it helpful. When sugar is dissolved in water,. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.youtube.com
Calculate the molarity of NaOH in the solution prepared by dissolving What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture I think it is equal because if 20 ml sugar and 50 ml. The sugar adds about 9/16 of. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. We have to calculate the volume of water before we dilution the question. The initial concentration is 25%, the final. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.
From www.chegg.com
Solved 16. Honey is a solution consisting almost entirely of What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture We have to calculate the volume of water before we dilution the question. The initial concentration is 25%, the final concentration is. However, when mixing miscible liquids (such as water and ethanol), the final volume of solution is not exactly equal to the. I think it is equal because if 20 ml sugar and 50 ml. When sugar is dissolved. What Is The Volume Of The 20 Ml Sugar And 50 Ml Water Mixture.