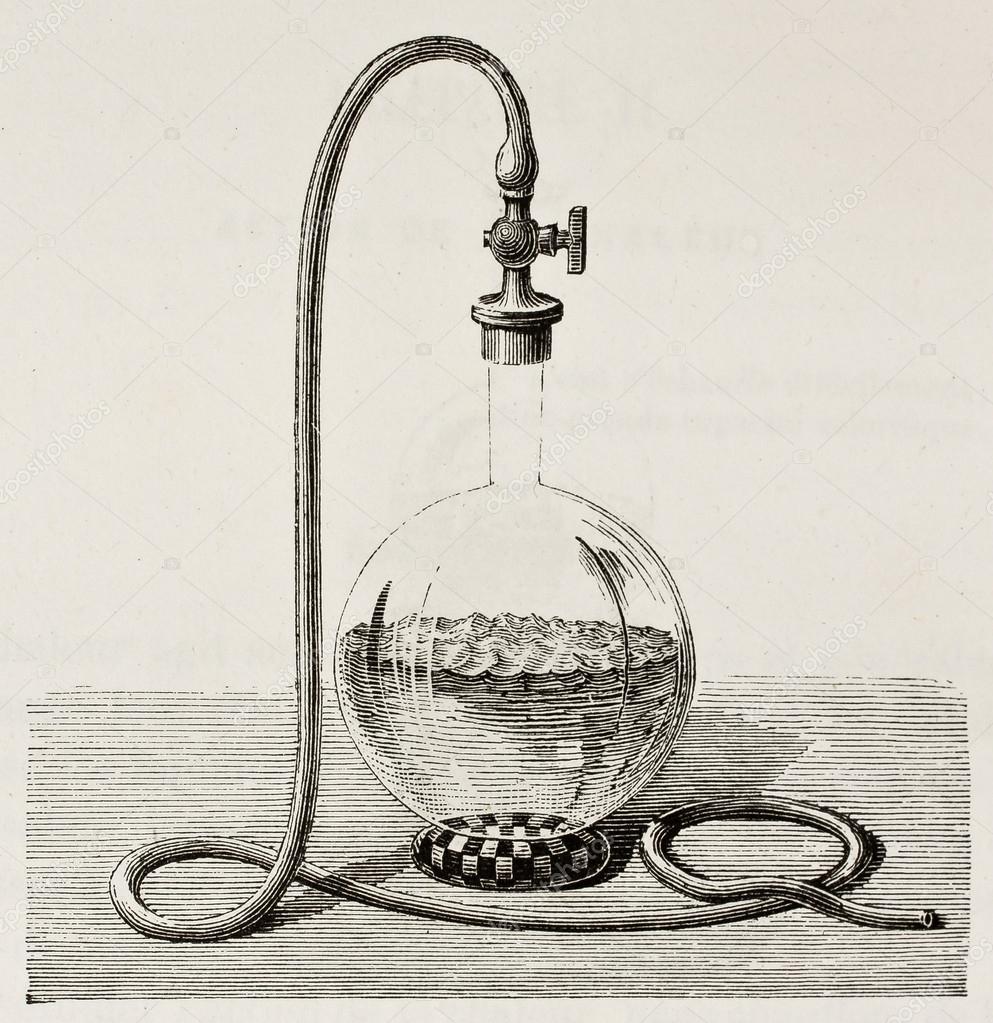
Water Boiling Under Vacuum . This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. Solutes can elevate the boiling point. When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. When water is heated up under vacuum, its boiling point is lower than at atmospheric. The opposite effect occurs when you. In a vacuum chamber, water can boil at room temperature. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. What is the boiling point of water at low pressure ? 95 rows water boiling point at vacuum pressure. Learn how the vapor pressure of water.
from depositphotos.com
Learn how the vapor pressure of water. 95 rows water boiling point at vacuum pressure. In a vacuum chamber, water can boil at room temperature. This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. Solutes can elevate the boiling point. What is the boiling point of water at low pressure ? Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. The opposite effect occurs when you. When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. When water is heated up under vacuum, its boiling point is lower than at atmospheric.
Boiling under vacuum — Stock Photo © marzolino 12311239
Water Boiling Under Vacuum In a vacuum chamber, water can boil at room temperature. 95 rows water boiling point at vacuum pressure. In a vacuum chamber, water can boil at room temperature. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. Learn how the vapor pressure of water. When water is heated up under vacuum, its boiling point is lower than at atmospheric. What is the boiling point of water at low pressure ? When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. Solutes can elevate the boiling point. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. The opposite effect occurs when you.
From www.youtube.com
Boiling Point and Pressure Boiling Water With Ice and In a Vacuum Pump Water Boiling Under Vacuum This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. When water is heated up under vacuum, its boiling point is lower than at atmospheric. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,.. Water Boiling Under Vacuum.
From future4200.com
Understanding vacuum and boiling point relationship Distillation Water Boiling Under Vacuum Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. In a vacuum chamber, water can boil at room temperature. When we reduce the pressure. Water Boiling Under Vacuum.
From www.youtube.com
Does Water Really Boil in a Vacuum Chamber? And Why? YouTube Water Boiling Under Vacuum When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. Learn how the vapor pressure of water. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. Solutes can elevate the boiling point. 95 rows water boiling point at vacuum pressure. Figures and tables showing how the properties of. Water Boiling Under Vacuum.
From www.youtube.com
Why does water boil in a vacuum? YouTube Water Boiling Under Vacuum In a vacuum chamber, water can boil at room temperature. The opposite effect occurs when you. Solutes can elevate the boiling point. 95 rows water boiling point at vacuum pressure. What is the boiling point of water at low pressure ? Learn how the vapor pressure of water. Figures and tables showing how the properties of water changes along the. Water Boiling Under Vacuum.
From bceweb.org
Boiling Point Under Vacuum Chart A Visual Reference of Charts Chart Water Boiling Under Vacuum This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. Solutes can elevate the boiling point. When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. What is the boiling point of water at low pressure ? The opposite effect occurs when you. In a. Water Boiling Under Vacuum.
From www.youtube.com
Water Boiling in a Vacuum Chamber YouTube Water Boiling Under Vacuum The opposite effect occurs when you. What is the boiling point of water at low pressure ? 28 rows find the boiling point of water at different pressures and vacuum levels in this table. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. Learn how. Water Boiling Under Vacuum.
From vacaero.com
Simple Physics for the High Vacuum Processing Industry Water Boiling Under Vacuum Solutes can elevate the boiling point. What is the boiling point of water at low pressure ? When water is heated up under vacuum, its boiling point is lower than at atmospheric. Learn how the vapor pressure of water. In a vacuum chamber, water can boil at room temperature. 95 rows water boiling point at vacuum pressure. The opposite effect. Water Boiling Under Vacuum.
From www.youtube.com
Vacuum pump boiling water at room temperature YouTube Water Boiling Under Vacuum What is the boiling point of water at low pressure ? When water is heated up under vacuum, its boiling point is lower than at atmospheric. Learn how the vapor pressure of water. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. The opposite effect. Water Boiling Under Vacuum.
From www.youtube.com
Vapor Pressure and Boiling YouTube Water Boiling Under Vacuum What is the boiling point of water at low pressure ? The opposite effect occurs when you. This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. 28 rows find the boiling point of water. Water Boiling Under Vacuum.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Water Boiling Under Vacuum When water is heated up under vacuum, its boiling point is lower than at atmospheric. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. Solutes can elevate the boiling point. This effectively increases the boiling point of the liquid and it takes a higher temperature. Water Boiling Under Vacuum.
From www.sciencelearn.org.nz
A pot of boiling water — Science Learning Hub Water Boiling Under Vacuum This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. What is the boiling point of water at low pressure ? When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. Solutes can elevate the boiling point. Learn how the vapor pressure of water. 95. Water Boiling Under Vacuum.
From www.youtube.com
Cold water boiling under vacuum! YouTube Water Boiling Under Vacuum The opposite effect occurs when you. When water is heated up under vacuum, its boiling point is lower than at atmospheric. What is the boiling point of water at low pressure ? 28 rows find the boiling point of water at different pressures and vacuum levels in this table. Solutes can elevate the boiling point. 95 rows water boiling point. Water Boiling Under Vacuum.
From www.researchgate.net
Vacuum pressure (represented as negative values) needed for boiling Water Boiling Under Vacuum This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. What is the boiling point of water at low pressure ? When water is heated up under vacuum, its boiling point is lower than at. Water Boiling Under Vacuum.
From www.youtube.com
Estimation of Solvent Boiling Points based on Vacuum YouTube Water Boiling Under Vacuum 28 rows find the boiling point of water at different pressures and vacuum levels in this table. Solutes can elevate the boiling point. The opposite effect occurs when you. What is the boiling point of water at low pressure ? When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. Learn how the vapor pressure of. Water Boiling Under Vacuum.
From gfecc.org
Gallery of liquid ring vacuum pump working principle and pumping system Water Boiling Under Vacuum When water is heated up under vacuum, its boiling point is lower than at atmospheric. The opposite effect occurs when you. Learn how the vapor pressure of water. This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. What is the boiling point of water at low pressure ?. Water Boiling Under Vacuum.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Water Boiling Under Vacuum This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. 95 rows water boiling point at vacuum pressure. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density,. Water Boiling Under Vacuum.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Water Boiling Under Vacuum Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. 28 rows find the boiling point of water at different pressures and vacuum levels in. Water Boiling Under Vacuum.
From www.youtube.com
Boiling Water In A Vacuum YouTube Water Boiling Under Vacuum In a vacuum chamber, water can boil at room temperature. When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. When water is heated up under vacuum, its boiling point is lower than at atmospheric. What is the boiling. Water Boiling Under Vacuum.
From scindustrialsales.com
Boiling Point of Water Under Vacuum Pumps and Filtration Water Boiling Under Vacuum When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. When water is heated up under vacuum, its boiling point is lower than at atmospheric. Learn how the vapor pressure of water. Figures and tables showing how the properties. Water Boiling Under Vacuum.
From www.researchgate.net
Boiling point distribution curves for the two vacuum distillate samples Water Boiling Under Vacuum The opposite effect occurs when you. Learn how the vapor pressure of water. What is the boiling point of water at low pressure ? Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. This effectively increases the boiling point of the liquid and it takes. Water Boiling Under Vacuum.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Water Boiling Under Vacuum Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the. Water Boiling Under Vacuum.
From www.youtube.com
STATES OF MATTER boiling water vacuum YouTube Water Boiling Under Vacuum When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. What is the boiling point of water at low pressure ? 28 rows find the boiling point of water at different pressures and vacuum levels in this table. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. Water Boiling Under Vacuum.
From mungfali.com
Water Boiling Point Pressure Chart Water Boiling Under Vacuum This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. The opposite effect occurs when you. Learn how the vapor pressure of water. In a vacuum chamber, water can boil at room temperature. 95 rows water boiling point at vacuum pressure. Solutes can elevate the boiling point. Figures and. Water Boiling Under Vacuum.
From www.youtube.com
??으로 물 끓이기, 대기압,진공,진공실험,도박사의 물리실험,Boiling water with vacuum Water Boiling Under Vacuum When water is heated up under vacuum, its boiling point is lower than at atmospheric. In a vacuum chamber, water can boil at room temperature. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. Solutes can elevate the boiling point. The opposite effect occurs when you. Figures and tables showing how the. Water Boiling Under Vacuum.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Water Boiling Under Vacuum Learn how the vapor pressure of water. What is the boiling point of water at low pressure ? Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. The opposite effect occurs when you. In a vacuum chamber, water can boil at room temperature. This effectively. Water Boiling Under Vacuum.
From sciencenotes.org
How to Boil Water at Room Temperature Water Boiling Under Vacuum 95 rows water boiling point at vacuum pressure. Learn how the vapor pressure of water. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. In a vacuum chamber, water can boil at room temperature. This effectively increases the boiling point of the liquid and it. Water Boiling Under Vacuum.
From mavink.com
Vacuum Boiling Point Chart Water Boiling Under Vacuum This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. Solutes can elevate the boiling point. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. The opposite effect occurs when you. 95 rows water boiling point at vacuum pressure. In a. Water Boiling Under Vacuum.
From www.researchgate.net
acuum drying seeks to reduce the boiling point of water in order to Water Boiling Under Vacuum 95 rows water boiling point at vacuum pressure. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. Solutes can elevate the boiling point. The opposite effect occurs when. Water Boiling Under Vacuum.
From www.youtube.com
Boiling Water via Vacuum Chamber YouTube Water Boiling Under Vacuum Learn how the vapor pressure of water. When water is heated up under vacuum, its boiling point is lower than at atmospheric. 95 rows water boiling point at vacuum pressure. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. In a vacuum chamber, water can boil at room temperature. This effectively increases. Water Boiling Under Vacuum.
From www.youtube.com
Boiling water freezes in vacuum YouTube Water Boiling Under Vacuum In a vacuum chamber, water can boil at room temperature. 28 rows find the boiling point of water at different pressures and vacuum levels in this table. Learn how the vapor pressure of water. 95 rows water boiling point at vacuum pressure. Solutes can elevate the boiling point. When we reduce the pressure surrounding water to below atmospheric levels, we. Water Boiling Under Vacuum.
From www.youtube.com
Boiling Point Under Vacuum Detailed Explanations YouTube Water Boiling Under Vacuum In a vacuum chamber, water can boil at room temperature. What is the boiling point of water at low pressure ? When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. 95 rows water boiling point at vacuum pressure. 28 rows find the boiling point of water at different pressures and vacuum levels in this table.. Water Boiling Under Vacuum.
From www.youtube.com
boiling water in vacuum chamber YouTube Water Boiling Under Vacuum This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. The opposite effect occurs when you. Learn how the vapor pressure of water. When water is heated up under vacuum, its boiling point is lower than at atmospheric. In a vacuum chamber, water can boil at room temperature. 28. Water Boiling Under Vacuum.
From depositphotos.com
Boiling under vacuum — Stock Photo © marzolino 12311239 Water Boiling Under Vacuum The opposite effect occurs when you. Learn how the vapor pressure of water. When water is heated up under vacuum, its boiling point is lower than at atmospheric. When we reduce the pressure surrounding water to below atmospheric levels, we create vacuum. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. Water Boiling Under Vacuum.
From sciencenotes.org
How to Boil Water at Room Temperature Water Boiling Under Vacuum 28 rows find the boiling point of water at different pressures and vacuum levels in this table. In a vacuum chamber, water can boil at room temperature. 95 rows water boiling point at vacuum pressure. Learn how the vapor pressure of water. This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all. Water Boiling Under Vacuum.
From www.youtube.com
Boiling cold water In a Vacuum Chamber YouTube Water Boiling Under Vacuum Solutes can elevate the boiling point. This effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number,. 95 rows water boiling point at vacuum pressure. When. Water Boiling Under Vacuum.