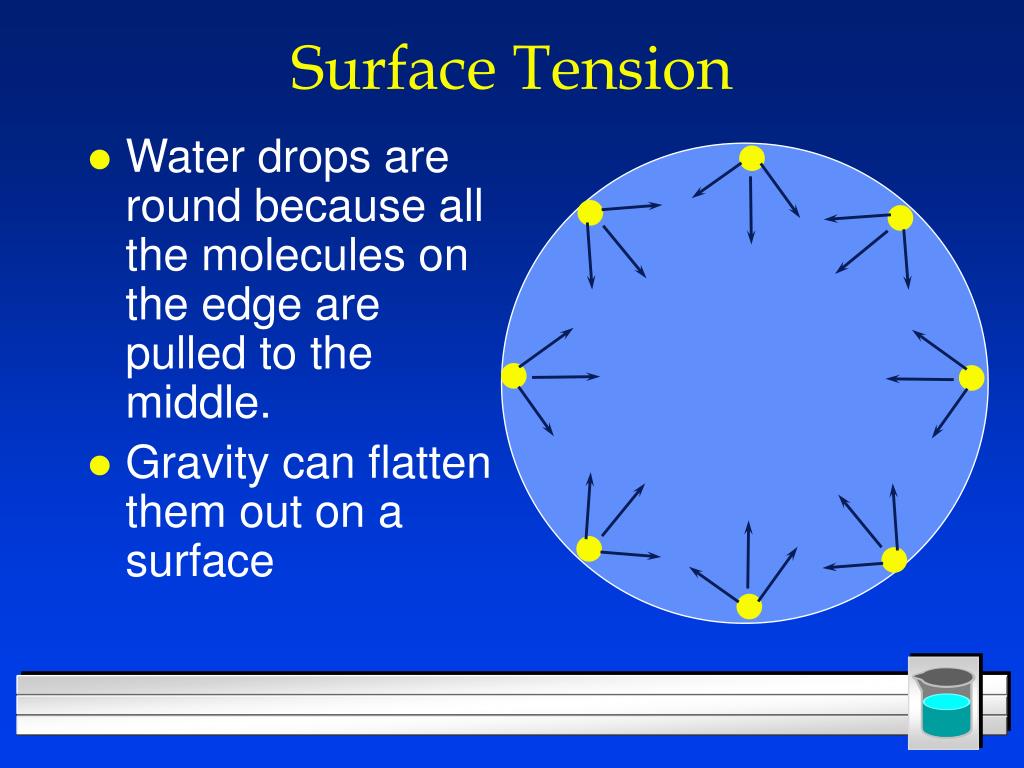
How Surface Tension Of Water . surface tension is caused by the inward attraction of molecules at a boundary. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. water has high surface tension, which can be explained by its polarity and hydrogen bonding. next to mercury, water has the highest surface tension of all commonly occurring liquids. Water consists of one oxygen atom flanked by. In comparison, organic liquids, such as benzene and alcohols ,. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn the surface tension of water and the formula to find the. Surface tension is a manifestation. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is the energy required to stretch a unit change of surface. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs.
from www.slideserve.com
Learn the surface tension of water and the formula to find the. Surface tension is a manifestation. next to mercury, water has the highest surface tension of all commonly occurring liquids. water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Surface tension is the energy required to stretch a unit change of surface. Water consists of one oxygen atom flanked by. In comparison, organic liquids, such as benzene and alcohols ,. surface tension is caused by the inward attraction of molecules at a boundary.
PPT Chapter 15 PowerPoint Presentation ID245553
How Surface Tension Of Water surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. In comparison, organic liquids, such as benzene and alcohols ,. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). water has high surface tension, which can be explained by its polarity and hydrogen bonding. Learn the surface tension of water and the formula to find the. surface tension is caused by the inward attraction of molecules at a boundary. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Surface tension is a manifestation. Water consists of one oxygen atom flanked by. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is the energy required to stretch a unit change of surface.
From wallpapercave.com
Surface Tension Water Wallpapers Wallpaper Cave How Surface Tension Of Water Water consists of one oxygen atom flanked by. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. next to mercury, water has the highest surface tension of all commonly occurring liquids. water has a surface tension of 0.07275 joule per. How Surface Tension Of Water.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Water consists of one oxygen atom flanked by. Learn the surface tension of water and the formula to find the.. How Surface Tension Of Water.
From pixels.com
Surface Tension Of Water Photograph by Dr Jeremy Burgess/science Photo How Surface Tension Of Water Learn the surface tension of water and the formula to find the. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is the energy required to stretch a unit change of surface. In comparison, organic liquids, such as benzene and alcohols ,. surface tension in water might be good. How Surface Tension Of Water.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How Surface Tension Of Water Surface tension is the energy required to stretch a unit change of surface. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk. How Surface Tension Of Water.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse How Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols ,. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation. Water consists of one oxygen atom flanked by. surface tension is caused by the inward attraction of molecules at a boundary. Learn the surface tension of water and the. How Surface Tension Of Water.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols ,. surface tension is caused by the inward attraction of molecules at a boundary. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Water consists of one oxygen atom flanked by. water has a surface. How Surface Tension Of Water.
From sciencewithkids.com
Water Surface Tension Experiment Science with How Surface Tension Of Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. surface tension is caused by. How Surface Tension Of Water.
From www.thoughtco.com
Liquid Definition and Examples (Chemistry) How Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension of water can cause things to float. How Surface Tension Of Water.
From studyfullirene.z21.web.core.windows.net
Surface Tension Of Water Worksheet How Surface Tension Of Water next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn the surface tension of water and the formula to find the. surface tension of water can cause things to float which are denser than water, allowing. How Surface Tension Of Water.
From errantscience.com
Water surface tension ErrantScience How Surface Tension Of Water next to mercury, water has the highest surface tension of all commonly occurring liquids. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by. Learn the surface tension of water and the formula to find the. surface tension of water can cause things to. How Surface Tension Of Water.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Surface Tension Of Water surface tension is caused by the inward attraction of molecules at a boundary. next to mercury, water has the highest surface tension of all commonly occurring liquids. Water consists of one oxygen atom flanked by. In comparison, organic liquids, such as benzene and alcohols ,. water has a surface tension of 0.07275 joule per square metre at. How Surface Tension Of Water.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Surface Tension Of Water surface tension is caused by the inward attraction of molecules at a boundary. In comparison, organic liquids, such as benzene and alcohols ,. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Water consists of one oxygen atom flanked by. surface tension in water. How Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 How Surface Tension Of Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. In comparison, organic liquids, such as benzene and alcohols ,. Surface tension is the energy required to stretch a unit change of surface. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn the surface tension. How Surface Tension Of Water.
From byjus.com
Explain the surface tension phenomenon with examples. How Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols ,. Water consists of one oxygen atom flanked by. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is caused by the inward attraction of molecules at a boundary. Surface tension is a manifestation. water has high surface tension,. How Surface Tension Of Water.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How Surface Tension Of Water Learn the surface tension of water and the formula to find the. surface tension is caused by the inward attraction of molecules at a boundary. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is the energy required to stretch a unit change of surface. water has high surface tension,. How Surface Tension Of Water.
From www.scienceabc.com
Why Does Water Feel Like Concrete When You Belly Flop Into It? » ScienceABC How Surface Tension Of Water Surface tension is the energy required to stretch a unit change of surface. In comparison, organic liquids, such as benzene and alcohols ,. Learn the surface tension of water and the formula to find the. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is the elastic tendency of fluid. How Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download How Surface Tension Of Water surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. surface tension is caused by the inward attraction of molecules at a boundary. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface,. How Surface Tension Of Water.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co How Surface Tension Of Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is the energy required. How Surface Tension Of Water.
From studylibrarytoweled.z13.web.core.windows.net
What Has Surface Tension How Surface Tension Of Water surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. In comparison, organic liquids, such as benzene and alcohols ,. Learn the surface tension of water and the formula to find the. Surface tension is a manifestation. surface tension is the elastic tendency of fluid surfaces. How Surface Tension Of Water.
From www.writework.com
Surface Tension WriteWork How Surface Tension Of Water Water consists of one oxygen atom flanked by. Surface tension is the energy required to stretch a unit change of surface. In comparison, organic liquids, such as benzene and alcohols ,. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation. Learn the surface tension of water and the formula. How Surface Tension Of Water.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments How Surface Tension Of Water Learn the surface tension of water and the formula to find the. Surface tension is a manifestation. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is caused by the inward attraction of molecules. How Surface Tension Of Water.
From www.thoughtco.com
Surface Tension Definition in Chemistry How Surface Tension Of Water Surface tension is the energy required to stretch a unit change of surface. Surface tension is a manifestation. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by. next to mercury, water has the highest surface tension of all commonly occurring liquids. Learn the. How Surface Tension Of Water.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols ,. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Learn the surface tension of water and the formula to find the. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. surface tension in water. How Surface Tension Of Water.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical How Surface Tension Of Water Surface tension is the energy required to stretch a unit change of surface. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Learn the. How Surface Tension Of Water.
From funsizephysics.com
What is Surface Tension? FunsizePhysics How Surface Tension Of Water next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is caused by the inward attraction of molecules at a boundary. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension in water might be good at performing tricks, such as being able. How Surface Tension Of Water.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii How Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is the energy required to stretch a unit change of surface. next to mercury, water has the highest surface tension of all commonly occurring liquids. water has high surface tension, which can be explained by its polarity and hydrogen. How Surface Tension Of Water.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How Surface Tension Of Water surface tension is caused by the inward attraction of molecules at a boundary. Water consists of one oxygen atom flanked by. next to mercury, water has the highest surface tension of all commonly occurring liquids. In comparison, organic liquids, such as benzene and alcohols ,. Surface tension is the energy required to stretch a unit change of surface.. How Surface Tension Of Water.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols ,. surface tension is caused by the inward attraction of molecules at a boundary. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation. Learn the surface tension of water and the formula to find the. surface. How Surface Tension Of Water.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download How Surface Tension Of Water surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation. surface tension is caused by the inward attraction of molecules. How Surface Tension Of Water.
From exoorzvyy.blob.core.windows.net
Surface Tension Of Water Lesson Plan at Sabrina Nickerson blog How Surface Tension Of Water surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Surface tension is a manifestation. next to mercury, water has the highest surface tension of all commonly occurring liquids. Learn the surface tension of water and the formula to find the. In comparison, organic liquids, such. How Surface Tension Of Water.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… How Surface Tension Of Water surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension. How Surface Tension Of Water.
From blog.biolinscientific.com
Why is surface tension important? How Surface Tension Of Water Water consists of one oxygen atom flanked by. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Learn the surface tension of water and the formula to find the. water has a surface tension of 0.07275 joule per square metre at. How Surface Tension Of Water.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Surface Tension Of Water surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn the surface tension of water and the formula to find the. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is the energy required to stretch a unit change of surface. water has. How Surface Tension Of Water.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? How Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. water has high surface tension, which can be explained by. How Surface Tension Of Water.
From www.biolinscientific.com
3 ways to measure surface tension How Surface Tension Of Water Surface tension is a manifestation. next to mercury, water has the highest surface tension of all commonly occurring liquids. Water consists of one oxygen atom flanked by. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Learn the surface tension of water and the formula to find the. In comparison, organic. How Surface Tension Of Water.