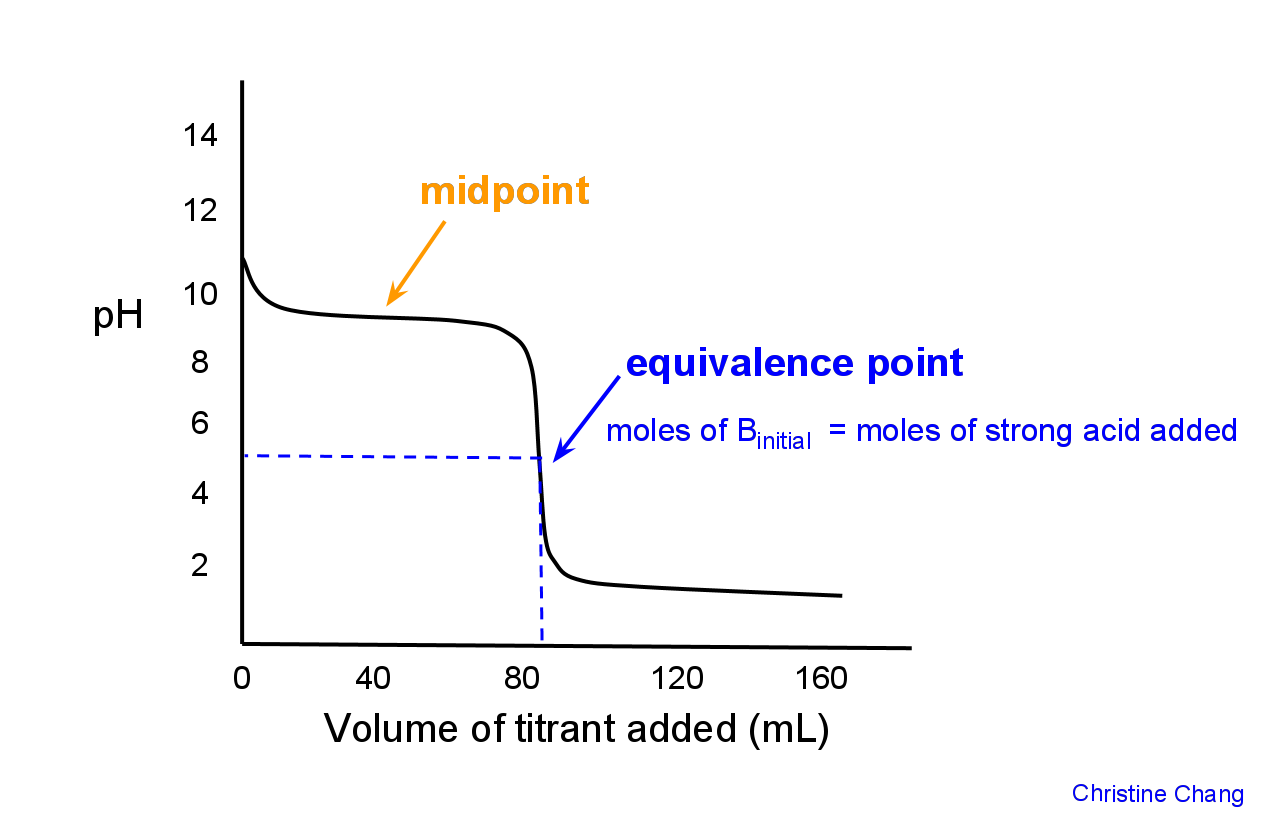
Titration Equivalence Point Weak Base . If you have a buffer remaining (the weak. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). titration curves for strong acid v strong base. one point in the titration of a weak acid or a weak base is particularly important: When solving a titration problem with a weak acid and a strong base there are certain values. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. the molarity of the acid is given, so the number of moles titrated can be calculated: We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. weak acid and strong base titration problems. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. 0.050 l × 6 mol/l = 0.3 moles.
from chem.libretexts.org
one point in the titration of a weak acid or a weak base is particularly important: We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. weak acid and strong base titration problems. titration curves for strong acid v strong base. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. 0.050 l × 6 mol/l = 0.3 moles. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. the molarity of the acid is given, so the number of moles titrated can be calculated: When solving a titration problem with a weak acid and a strong base there are certain values.
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts
Titration Equivalence Point Weak Base the molarity of the acid is given, so the number of moles titrated can be calculated: note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. one point in the titration of a weak acid or a weak base is particularly important: titration curves for strong acid v strong base. the molarity of the acid is given, so the number of moles titrated can be calculated: We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). When solving a titration problem with a weak acid and a strong base there are certain values. 0.050 l × 6 mol/l = 0.3 moles. If you have a buffer remaining (the weak. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. weak acid and strong base titration problems.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Equivalence Point Weak Base When solving a titration problem with a weak acid and a strong base there are certain values. weak acid and strong base titration problems. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. 0.050 l × 6 mol/l = 0.3 moles. (b). Titration Equivalence Point Weak Base.
From giorvlvoc.blob.core.windows.net
Acid Base Titration Curve at Adrian Yount blog Titration Equivalence Point Weak Base 0.050 l × 6 mol/l = 0.3 moles. If you have a buffer remaining (the weak. one point in the titration of a weak acid or a weak base is particularly important: note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. (b) the. Titration Equivalence Point Weak Base.
From gioxrzofe.blob.core.windows.net
Calculation Formula For Titration at Allison Rosario blog Titration Equivalence Point Weak Base titration curves for strong acid v strong base. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). one point in the titration of a weak acid or a weak base is particularly important: note that the ph at the equivalence point of this titration is significantly greater. Titration Equivalence Point Weak Base.
From www.chegg.com
Solved 10. Explain why the pH titration curve shown below Titration Equivalence Point Weak Base in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. titration curves for strong acid v strong base. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). When solving a titration problem with a. Titration Equivalence Point Weak Base.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid Titration Equivalence Point Weak Base titration curves for strong acid v strong base. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). in. Titration Equivalence Point Weak Base.
From mavink.com
Strong Acid And Strong Base Titration Curve Titration Equivalence Point Weak Base If you have a buffer remaining (the weak. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. note that the ph at the equivalence point of this titration is significantly greater than. Titration Equivalence Point Weak Base.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Equivalence Point Weak Base in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. If you have a buffer remaining (the weak. one point in the titration of a weak acid or a weak base is particularly important: 0.050 l × 6 mol/l = 0.3 moles. weak. Titration Equivalence Point Weak Base.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Equivalence Point Weak Base one point in the titration of a weak acid or a weak base is particularly important: note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. weak acid and strong base titration problems. We'll take hydrochloric acid and sodium hydroxide as typical of a. Titration Equivalence Point Weak Base.
From hxecuzoly.blob.core.windows.net
Difference Between Half Equivalence Point And Equivalence Point at Titration Equivalence Point Weak Base (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. the molarity of the acid. Titration Equivalence Point Weak Base.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Equivalence Point Weak Base note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. If you have a buffer remaining. Titration Equivalence Point Weak Base.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Titration Equivalence Point Weak Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). the molarity of the acid is given, so the number of moles titrated can be calculated: When solving a titration problem with a. Titration Equivalence Point Weak Base.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Equivalence Point Weak Base (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. We'll take hydrochloric acid and sodium. Titration Equivalence Point Weak Base.
From www.slideshare.net
pH Understanding titration curve Titration Equivalence Point Weak Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. calculate the ph for the weak acid/strong base titration between 50.0 ml. Titration Equivalence Point Weak Base.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Titration Equivalence Point Weak Base 0.050 l × 6 mol/l = 0.3 moles. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). If you have a buffer remaining (the weak. weak acid and strong base titration problems. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly. Titration Equivalence Point Weak Base.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Equivalence Point Weak Base calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). If you have a buffer remaining (the weak. the molarity of the acid is given, so the number of moles titrated can be calculated: one point in the titration of a weak acid or a weak base is particularly. Titration Equivalence Point Weak Base.
From hxehjedhh.blob.core.windows.net
Titration Notes Grade 12 Pdf at Erica Casella blog Titration Equivalence Point Weak Base calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). weak acid and strong base titration problems. 0.050 l × 6 mol/l = 0.3 moles. note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. If. Titration Equivalence Point Weak Base.
From exoaqbcwf.blob.core.windows.net
Equivalence Point Titration With Base at Alvin Barajas blog Titration Equivalence Point Weak Base note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. the molarity of the acid. Titration Equivalence Point Weak Base.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Equivalence Point Weak Base one point in the titration of a weak acid or a weak base is particularly important: If you have a buffer remaining (the weak. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with. Titration Equivalence Point Weak Base.
From www.youtube.com
Titration Weak base/Strong acid Equivalence Point YouTube Titration Equivalence Point Weak Base one point in the titration of a weak acid or a weak base is particularly important: note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. weak acid and strong base titration problems. When solving a titration problem with a weak acid and a. Titration Equivalence Point Weak Base.
From mungfali.com
Titration Curve Labeled Titration Equivalence Point Weak Base weak acid and strong base titration problems. When solving a titration problem with a weak acid and a strong base there are certain values. titration curves for strong acid v strong base. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence. Titration Equivalence Point Weak Base.
From giosbxlyr.blob.core.windows.net
Ph Titration Curve Equivalence Point at Kimberly Hunter blog Titration Equivalence Point Weak Base When solving a titration problem with a weak acid and a strong base there are certain values. the molarity of the acid is given, so the number of moles titrated can be calculated: note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. titration. Titration Equivalence Point Weak Base.
From www.youtube.com
Titration Weak base/strong acid Before the equivalence point YouTube Titration Equivalence Point Weak Base If you have a buffer remaining (the weak. When solving a titration problem with a weak acid and a strong base there are certain values. 0.050 l × 6 mol/l = 0.3 moles. titration curves for strong acid v strong base. the molarity of the acid is given, so the number of moles titrated can be calculated: . Titration Equivalence Point Weak Base.
From www.youtube.com
Weak acid / strong base titration pH after equivalence point YouTube Titration Equivalence Point Weak Base in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. When solving a titration problem with a weak acid and a strong base there are certain values. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic. Titration Equivalence Point Weak Base.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Titration Equivalence Point Weak Base 0.050 l × 6 mol/l = 0.3 moles. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. the molarity of the acid is given, so the number of moles titrated can be calculated: We'll take hydrochloric acid and sodium hydroxide as typical of. Titration Equivalence Point Weak Base.
From hxeixtrjs.blob.core.windows.net
Difference Between Halfway Point And Equivalence Point at Jennifer Hay blog Titration Equivalence Point Weak Base in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. note that the ph at the equivalence point of this titration is significantly greater than 7, as. Titration Equivalence Point Weak Base.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Equivalence Point Weak Base weak acid and strong base titration problems. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). 0.050 l × 6 mol/l = 0.3 moles. one point in the titration of a weak acid or a weak base is particularly important: When solving a titration problem with a weak. Titration Equivalence Point Weak Base.
From hxeqgqbgy.blob.core.windows.net
Titration Particle Diagram at Maxine Hatch blog Titration Equivalence Point Weak Base (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. titration curves for strong acid v strong base. weak acid and strong base titration problems. one point in the titration of a weak acid or a weak. Titration Equivalence Point Weak Base.
From www.hotzxgirl.com
Titration Curve Weak Base With Strong Acid Hot Sex Picture Titration Equivalence Point Weak Base (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. When solving a titration problem with. Titration Equivalence Point Weak Base.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Titration Equivalence Point Weak Base When solving a titration problem with a weak acid and a strong base there are certain values. 0.050 l × 6 mol/l = 0.3 moles. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong. Titration Equivalence Point Weak Base.
From hxevboeea.blob.core.windows.net
Titration Naoh And Oxalic Acid at Mercedes Hager blog Titration Equivalence Point Weak Base calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). weak acid and strong base titration problems. 0.050 l × 6 mol/l = 0.3 moles. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. When solving a titration problem with a weak. Titration Equivalence Point Weak Base.
From boisestate.pressbooks.pub
14.7 AcidBase Titrations General Chemistry 1 & 2 Titration Equivalence Point Weak Base (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m naoh (strong base) has an equivalence point of 8.72 ph. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. If you have a buffer. Titration Equivalence Point Weak Base.
From philschatz.com
AcidBase Titrations · Chemistry Titration Equivalence Point Weak Base 0.050 l × 6 mol/l = 0.3 moles. weak acid and strong base titration problems. titration curves for strong acid v strong base. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong. Titration Equivalence Point Weak Base.
From giosbxlyr.blob.core.windows.net
Ph Titration Curve Equivalence Point at Kimberly Hunter blog Titration Equivalence Point Weak Base one point in the titration of a weak acid or a weak base is particularly important: the molarity of the acid is given, so the number of moles titrated can be calculated: If you have a buffer remaining (the weak. (b) the titration curve for the titration of 25.00 ml of 0.100 m hcl (strong acid) with. Titration Equivalence Point Weak Base.
From www.numerade.com
SOLVED Examine the following titration curve and answer the following Titration Equivalence Point Weak Base We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. titration curves for strong acid v strong base. note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. When solving a titration problem with a weak acid and. Titration Equivalence Point Weak Base.
From www.youtube.com
Determining the pH During a Strong AcidWeak Base Titration at Titration Equivalence Point Weak Base in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. titration curves for strong acid v strong base. note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid. weak. Titration Equivalence Point Weak Base.