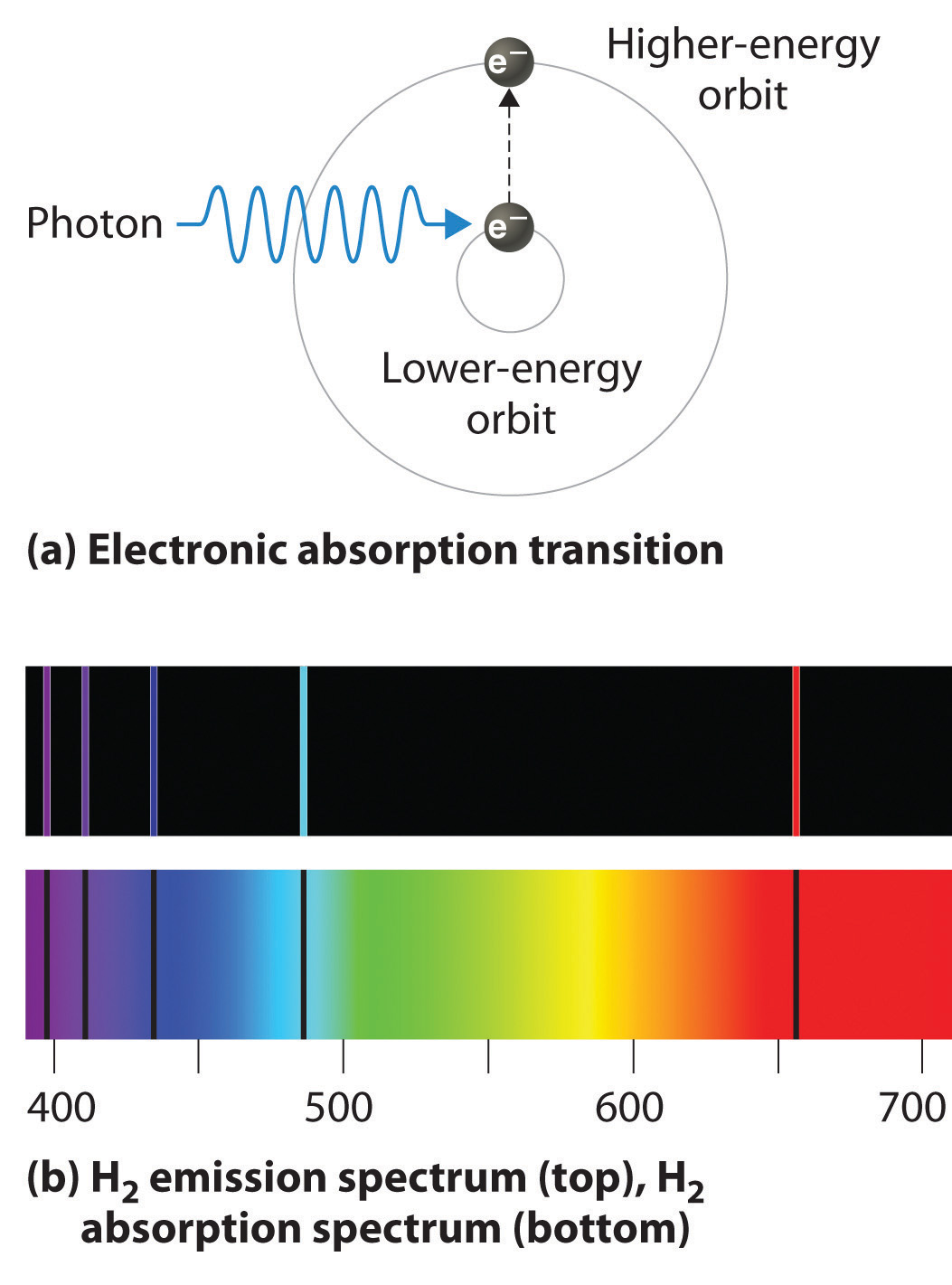
Emission Spectra Have Lines . A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A discontinuous spectrum consists of lines of specific wavelengths superimposed. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. The actual wavelengths of the lines are predictably.
from users.highland.edu
The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. A discontinuous spectrum consists of lines of specific wavelengths superimposed. The actual wavelengths of the lines are predictably. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains.
Atomic Spectra and Models of the Atom
Emission Spectra Have Lines A discontinuous spectrum consists of lines of specific wavelengths superimposed. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A discontinuous spectrum consists of lines of specific wavelengths superimposed. The actual wavelengths of the lines are predictably. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Have Lines When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The spectroscope separates the light of varying wavelengths. Emission Spectra Have Lines.
From winstonmcyponce.blogspot.com
Atomic Emission Spectrum of Hydrogen WinstonmcyPonce Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When the emitted light. Emission Spectra Have Lines.
From www.alamy.com
HHeHg emission spectra. Graphical representation of the emission Emission Spectra Have Lines A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A discontinuous spectrum consists. Emission Spectra Have Lines.
From pixels.com
Helium Emission And Absorption Spectra Photograph by Carlos Clarivan Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A discontinuous spectrum consists of lines of specific wavelengths superimposed. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. A continuous spectrum shows a smooth range of all. Emission Spectra Have Lines.
From www.visionlearning.com
History of Earth's Atmosphere I Earth Science Visionlearning Emission Spectra Have Lines A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called. Emission Spectra Have Lines.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. A discontinuous spectrum consists of lines of specific wavelengths superimposed. When. Emission Spectra Have Lines.
From www.youtube.com
Emission spectrum of hydrogen Chemistry Khan Academy YouTube Emission Spectra Have Lines The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. The actual wavelengths of the lines. Emission Spectra Have Lines.
From users.highland.edu
Atomic Spectra and Models of the Atom Emission Spectra Have Lines The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. A discontinuous spectrum consists of lines of specific wavelengths superimposed. When the emitted light is passed through a prism, only a few narrow. Emission Spectra Have Lines.
From mungfali.com
Atomic Emission Spectrum Of Elements Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of. Emission Spectra Have Lines.
From sixdayscience.com
Chapter 7 SixDay Science Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. A discontinuous spectrum consists of lines of specific wavelengths superimposed. When the emitted light is passed through a. Emission Spectra Have Lines.
From byjus.com
Emission spectrum Atomic spectra Chemistry Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A discontinuous spectrum consists of lines of specific wavelengths superimposed. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed. Emission Spectra Have Lines.
From cooptata.weebly.com
Atomic emission spectrum vs continuous spectrum cooptata Emission Spectra Have Lines A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. A discontinuous spectrum consists of lines of specific wavelengths superimposed. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When the emitted light is passed through a. Emission Spectra Have Lines.
From imagine.gsfc.nasa.gov
Spectra Introduction Emission Spectra Have Lines A discontinuous spectrum consists of lines of specific wavelengths superimposed. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps.. Emission Spectra Have Lines.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Spectra Have Lines A discontinuous spectrum consists of lines of specific wavelengths superimposed. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the. Emission Spectra Have Lines.
From www.thoughtco.com
Balmer Series Definition in Science Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a. Emission Spectra Have Lines.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Emission Spectra Have Lines When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. A discontinuous spectrum consists of lines of specific wavelengths superimposed. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light. Emission Spectra Have Lines.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectra Have Lines The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When the emitted light is passed through a prism, only a. Emission Spectra Have Lines.
From commons.wikimedia.org
FileAtomic emission spectrum of helium.svg Wikimedia Commons Emission Spectra Have Lines The spectroscope separates the light of varying wavelengths and we observe a line spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a. Emission Spectra Have Lines.
From www.slideserve.com
PPT EMISSION SPECTRUM PowerPoint Presentation, free download ID5777174 Emission Spectra Have Lines A discontinuous spectrum consists of lines of specific wavelengths superimposed. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. An atomic emission spectrum is the pattern of lines formed. Emission Spectra Have Lines.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A discontinuous spectrum consists of lines of specific wavelengths superimposed. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. When. Emission Spectra Have Lines.
From chemcollective.org
CHEM1315 Lab 8 Atomic Spectrum Emission Spectra Have Lines When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A continuous spectrum shows a smooth range of. Emission Spectra Have Lines.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra Have Lines The actual wavelengths of the lines are predictably. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. The spectroscope separates the light of varying wavelengths and we observe a. Emission Spectra Have Lines.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Have Lines The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the. Emission Spectra Have Lines.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Emission Spectra Have Lines A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. A discontinuous spectrum consists of lines of specific wavelengths superimposed. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably.. Emission Spectra Have Lines.
From www.slideserve.com
PPT Electrons and Periodicity PowerPoint Presentation, free download Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. The actual wavelengths of the lines are predictably.. Emission Spectra Have Lines.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances Emission Spectra Have Lines A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A discontinuous spectrum consists of lines of specific wavelengths superimposed. The spectroscope separates the light of varying wavelengths. Emission Spectra Have Lines.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra Have Lines When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. A discontinuous spectrum consists of lines of specific wavelengths superimposed. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light. Emission Spectra Have Lines.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Emission Spectra Have Lines The actual wavelengths of the lines are predictably. A discontinuous spectrum consists of lines of specific wavelengths superimposed. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. An atomic. Emission Spectra Have Lines.
From www.hanlin.com
Edexcel A Level Physics复习笔记5.38 Atomic Line Spectra翰林国际教育 Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When the emitted light is passed through a prism, only a. Emission Spectra Have Lines.
From www.pinterest.jp
Emission Spectra of the Elements!! Chemistry classroom, Teaching Emission Spectra Have Lines A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. A discontinuous spectrum consists of lines of specific wavelengths superimposed. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When the emitted light is passed through a. Emission Spectra Have Lines.
From www.pinterest.com
Image of absorption, emission, and continuous spectra. Absorption Emission Spectra Have Lines The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. A discontinuous spectrum consists of lines of specific wavelengths superimposed. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. An atomic emission. Emission Spectra Have Lines.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Have Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The actual wavelengths of the lines are predictably. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. The spectroscope separates the light of varying wavelengths and we. Emission Spectra Have Lines.
From scisyn.com
Spectra Examples Emission Spectra Have Lines When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. A discontinuous spectrum consists of lines of specific wavelengths superimposed. A continuous spectrum. Emission Spectra Have Lines.
From brainly.com
What is an emission spectrum? Use the Bohr model to explain why the Emission Spectra Have Lines A discontinuous spectrum consists of lines of specific wavelengths superimposed. A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum,. Emission Spectra Have Lines.
From www.researchgate.net
The origins of three of the major sodium emission spectral lines (the Emission Spectra Have Lines A continuous spectrum shows a smooth range of all wavelengths of light with no gaps. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. A discontinuous spectrum consists of lines of specific wavelengths superimposed. An atomic emission spectrum is the pattern of lines formed when light passes. Emission Spectra Have Lines.