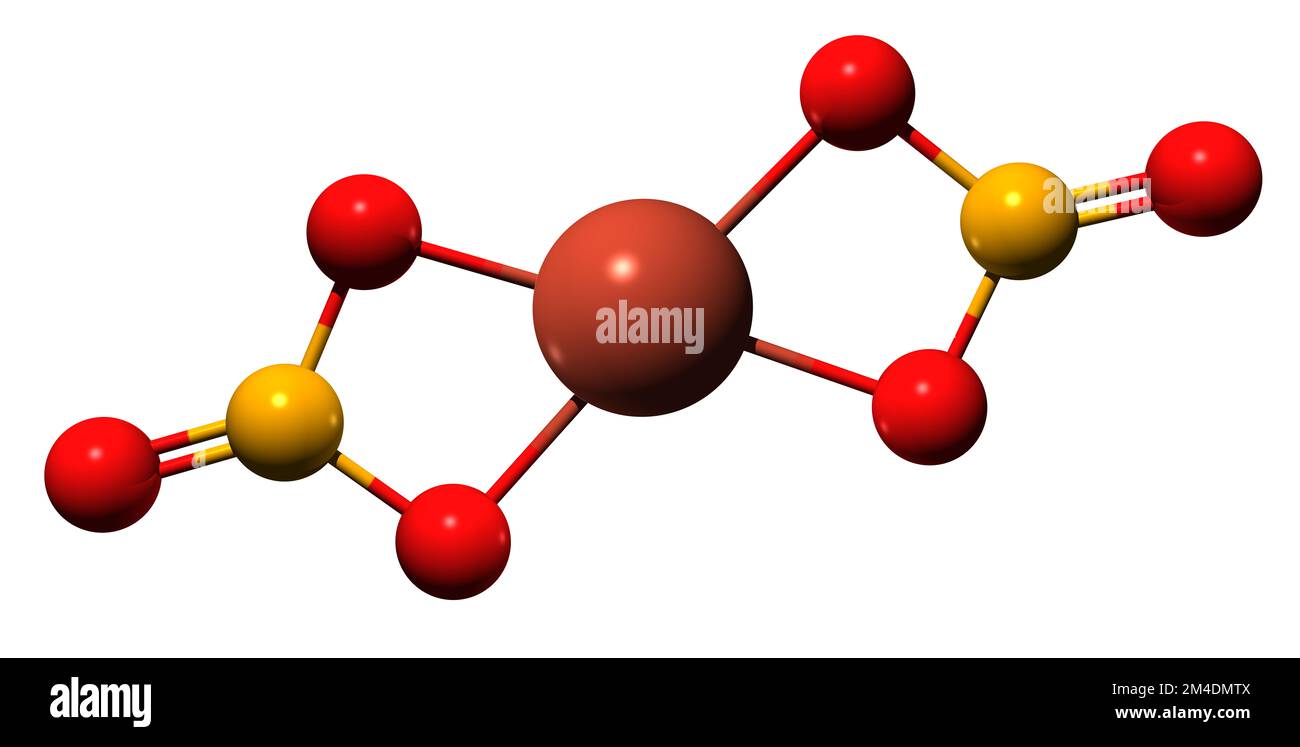
Copper Ii Nitrate State At Room Temperature . copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. what happens when copper nitrate undergoes heating? (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Copper nitrate cu no 3 2 is. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). copper (ii) nitrate.
from www.alamy.com
what happens when copper nitrate undergoes heating? copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. Copper nitrate cu no 3 2 is. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). copper (ii) nitrate. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate.
3D image of Copper II nitrate skeletal formula molecular chemical
Copper Ii Nitrate State At Room Temperature copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. copper (ii) nitrate. what happens when copper nitrate undergoes heating? Copper nitrate cu no 3 2 is. Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate.
From www.researchgate.net
Amount of copper nitrate in grams dissolved in 100 g of water and Copper Ii Nitrate State At Room Temperature Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). Copper nitrate cu no 3 2 is. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is. Copper Ii Nitrate State At Room Temperature.
From www.fishersci.dk
Copper(II) nitrate hemi(pentahydrate), 98, Thermo Scientific Chemicals Copper Ii Nitrate State At Room Temperature copper (ii) nitrate. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. what happens when copper. Copper Ii Nitrate State At Room Temperature.
From exoszvpor.blob.core.windows.net
Copper Ii Nitrate Colour at Ashley Jones blog Copper Ii Nitrate State At Room Temperature Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. copper (ii) nitrate. what happens when copper nitrate undergoes heating? Except where otherwise noted, data are given for materials. Copper Ii Nitrate State At Room Temperature.
From www.sciencephoto.com
Conductivity of Copper (II) Nitrate Stock Image C002/7953 Science Copper Ii Nitrate State At Room Temperature Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. copper (ii) nitrate. what happens when copper nitrate undergoes heating? copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. Except where otherwise noted, data are given for materials. Copper Ii Nitrate State At Room Temperature.
From cymitquimica.com
Copper(II) nitrate hydrate 3DFC45436 CymitQuimica Copper Ii Nitrate State At Room Temperature Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric. Copper Ii Nitrate State At Room Temperature.
From www.chegg.com
Solved 4. Copper(II) nitrate on heating according Copper Ii Nitrate State At Room Temperature what happens when copper nitrate undergoes heating? Copper nitrate cu no 3 2 is. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77. Copper Ii Nitrate State At Room Temperature.
From www.researchgate.net
Absorption spectrums of composite thin films using copper (II) nitrate Copper Ii Nitrate State At Room Temperature Copper nitrate cu no 3 2 is. copper (ii) nitrate. Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. Except where otherwise noted, data are given for materials in. Copper Ii Nitrate State At Room Temperature.
From testbook.com
Copper (II) Nitrate Formula Learn Structure, Properties, Uses Copper Ii Nitrate State At Room Temperature copper (ii) nitrate. Copper nitrate cu no 3 2 is. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Explore. Copper Ii Nitrate State At Room Temperature.
From www.youtube.com
HOW TO MAKE COPPER (II) NITRATE YouTube Copper Ii Nitrate State At Room Temperature copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. copper (ii) nitrate. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Except where otherwise noted, data. Copper Ii Nitrate State At Room Temperature.
From www.sciencephoto.com
Copper(II) nitrate Stock Image C043/2658 Science Photo Library Copper Ii Nitrate State At Room Temperature Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate,. Copper Ii Nitrate State At Room Temperature.
From www.youtube.com
On heating blue coloured powder of copper (II) nitrate in a boiling Copper Ii Nitrate State At Room Temperature (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Copper nitrate cu no 3 2 is. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. Except where. Copper Ii Nitrate State At Room Temperature.
From www.researchgate.net
Thermal of copper (II) nitrate trihydrate a DTA/TG Copper Ii Nitrate State At Room Temperature Copper nitrate cu no 3 2 is. copper (ii) nitrate. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. what happens when copper. Copper Ii Nitrate State At Room Temperature.
From www.youtube.com
On heating blue coloured powder of copper (II) nitrate in a boiling Copper Ii Nitrate State At Room Temperature Copper nitrate cu no 3 2 is. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Explore the. Copper Ii Nitrate State At Room Temperature.
From www.flinnsci.com
Copper(II) Nitrate, Lab Grade, 500 g Flinn Scientific Copper Ii Nitrate State At Room Temperature what happens when copper nitrate undergoes heating? (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). Copper nitrate cu no. Copper Ii Nitrate State At Room Temperature.
From www.pinterest.com
Copper (II) Nitrate Trihydrate, 500g LabGrade The Curated Chemical Copper Ii Nitrate State At Room Temperature Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). copper (ii) nitrate. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. what happens when copper nitrate undergoes heating? Explore the properties, preparation, uses,. Copper Ii Nitrate State At Room Temperature.
From www.researchgate.net
The TGA and DTG curves for copper nitrate and cerium nitrate with PVP Copper Ii Nitrate State At Room Temperature Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. what happens when copper nitrate undergoes heating? (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. copper nitrate, with the chemical formula 2. Copper Ii Nitrate State At Room Temperature.
From www.sciencephoto.com
Copper(II) nitrate Stock Image C043/2653 Science Photo Library Copper Ii Nitrate State At Room Temperature copper (ii) nitrate. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). what happens when copper nitrate undergoes heating? Copper nitrate cu no 3 2 is. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid. Copper Ii Nitrate State At Room Temperature.
From www.toppr.com
On heating blue coloured powder of copper (II) nitrate in a boiling Copper Ii Nitrate State At Room Temperature Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. what happens when copper nitrate undergoes heating? (d) describe how you would prepare a pure. Copper Ii Nitrate State At Room Temperature.
From www.youtube.com
COPPER (II) NITRATE THE COMPLETE GUIDE YouTube Copper Ii Nitrate State At Room Temperature Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid. Copper Ii Nitrate State At Room Temperature.
From www.researchgate.net
FTIR spectra of (A) bis(ethylenediamine)copper(II) nitrate and (B Copper Ii Nitrate State At Room Temperature Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. copper (ii) nitrate. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Except where otherwise noted, data are given for materials in their standard. Copper Ii Nitrate State At Room Temperature.
From www.flinnsci.com
Flinn Chemicals, Copper(II) Nitrate Solution Copper Ii Nitrate State At Room Temperature (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. what happens when copper nitrate undergoes heating? copper (ii) nitrate. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa).. Copper Ii Nitrate State At Room Temperature.
From www.wikiwand.com
Copper(II) nitrate Wikiwand Copper Ii Nitrate State At Room Temperature Copper nitrate cu no 3 2 is. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). copper (ii) nitrate. what happens when copper nitrate. Copper Ii Nitrate State At Room Temperature.
From www.researchgate.net
Scheme 8. Thermal mechanism of copper(II) nitrate Copper Ii Nitrate State At Room Temperature (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. what happens when copper nitrate undergoes heating? copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. Copper. Copper Ii Nitrate State At Room Temperature.
From www.researchgate.net
EPR spectrum of copper(II) complexes in ammonium nitrate at room Copper Ii Nitrate State At Room Temperature what happens when copper nitrate undergoes heating? Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. Copper nitrate cu no 3 2 is. (d) describe how you would prepare a pure. Copper Ii Nitrate State At Room Temperature.
From pubs.rsc.org
Spectroscopy and photochemistry of copper nitrate clusters Physical Copper Ii Nitrate State At Room Temperature Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Copper nitrate cu no 3 2 is. copper nitrate, with the. Copper Ii Nitrate State At Room Temperature.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium Copper Ii Nitrate State At Room Temperature (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. Except where otherwise noted, data are given for materials. Copper Ii Nitrate State At Room Temperature.
From www.researchgate.net
FTIR spectra of (A) bis(ethylenediamine)copper(II) nitrate and (B Copper Ii Nitrate State At Room Temperature Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. what happens when copper nitrate undergoes heating? Explore the properties, preparation,. Copper Ii Nitrate State At Room Temperature.
From lab.honeywell.com
Copper(II) nitrate hemi(pentahydrate) 12837 Honeywell Research Copper Ii Nitrate State At Room Temperature Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. copper (ii) nitrate. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline solid at room. what happens when copper nitrate undergoes heating? Copper nitrate cu no 3 2 is. (d). Copper Ii Nitrate State At Room Temperature.
From www.youtube.com
On heating blue coloured powder of copper (II) nitrate in a boiling Copper Ii Nitrate State At Room Temperature Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. Except where otherwise noted, data are given for materials in their standard state (at 25 °c. Copper Ii Nitrate State At Room Temperature.
From www.sciencemadness.org
Copper(II) nitrate Sciencemadness Wiki Copper Ii Nitrate State At Room Temperature what happens when copper nitrate undergoes heating? Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. copper (ii) nitrate.. Copper Ii Nitrate State At Room Temperature.
From www.thermofisher.com
Copper(II) nitrate trihydrate, 99, pure, Thermo Scientific Chemicals Copper Ii Nitrate State At Room Temperature (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. copper (ii) nitrate. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). Explore the properties, preparation, uses, and safety measures. Copper Ii Nitrate State At Room Temperature.
From slideplayer.com
Living By Chemistry SECOND EDITION ppt download Copper Ii Nitrate State At Room Temperature Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. copper (ii) nitrate. copper nitrate, with the chemical formula 2 cu(no 3) 2, is an inorganic compound that is a blue, crystalline. Copper Ii Nitrate State At Room Temperature.
From www.southernbiological.com
Copper (II) Nitrate, 0.1M, Laboratory Grade Southern Biological Copper Ii Nitrate State At Room Temperature Copper nitrate cu no 3 2 is. copper (ii) nitrate. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. what happens when copper nitrate undergoes heating? copper nitrate, with the. Copper Ii Nitrate State At Room Temperature.
From www.carolina.com
Copper (II) Nitrate Solution, 0.1 M, Laboratory Grade, 500 mL Copper Ii Nitrate State At Room Temperature copper (ii) nitrate. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. copper nitrate, with the chemical formula 2. Copper Ii Nitrate State At Room Temperature.
From www.alamy.com
3D image of Copper II nitrate skeletal formula molecular chemical Copper Ii Nitrate State At Room Temperature Explore the properties, preparation, uses, and safety measures of copper (ii) nitrate, a vital compound in. (d) describe how you would prepare a pure dry sample of copper(ii) nitrate crystals in the laboratory using dilute nitric acid and solid copper( ii ) carbonate. copper (ii) nitrate. Copper nitrate cu no 3 2 is. copper nitrate, with the. Copper Ii Nitrate State At Room Temperature.