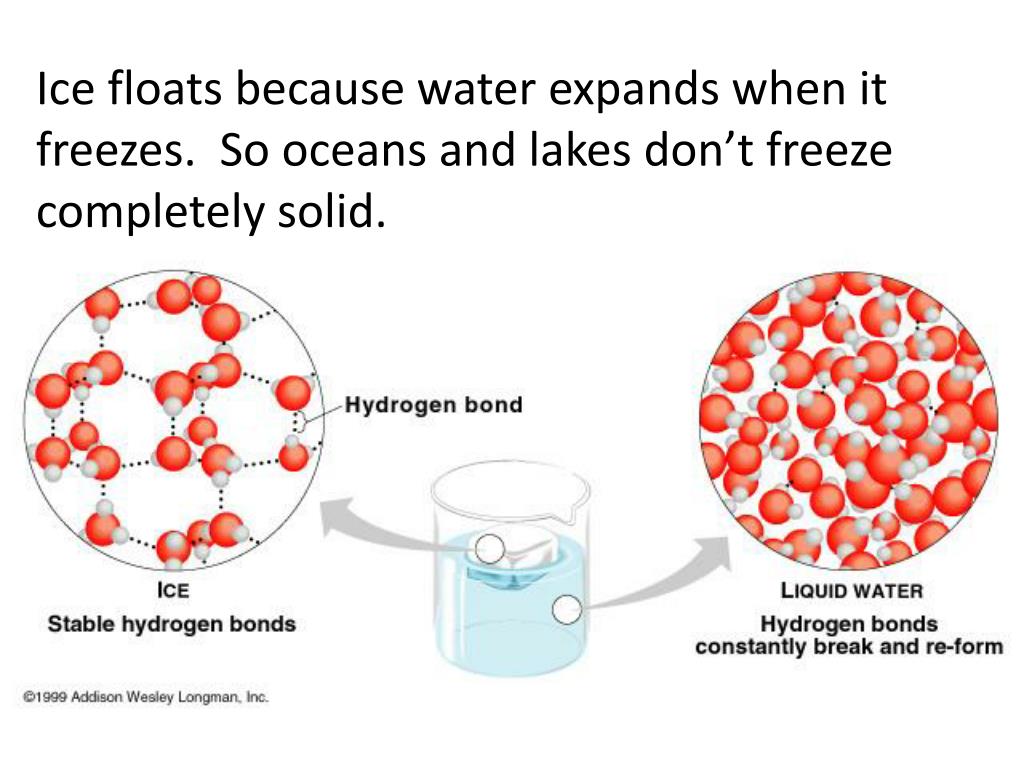
How Can Water Be Below Freezing And Not Freeze . By contrast, in moving water, the entire volume has to. Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. Is the freezing point the same as the melting point? Do you know the freezing point of water? if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. then, a thin layer at the surface starts to freeze while floating on the denser water below. the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze.
from www.slideserve.com
Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. Do you know the freezing point of water? In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze. now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. By contrast, in moving water, the entire volume has to. then, a thin layer at the surface starts to freeze while floating on the denser water below. we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. Is the freezing point the same as the melting point? if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal.
PPT Water, water everywhere! PowerPoint Presentation, free download
How Can Water Be Below Freezing And Not Freeze Do you know the freezing point of water? then, a thin layer at the surface starts to freeze while floating on the denser water below. Is the freezing point the same as the melting point? Do you know the freezing point of water? we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. By contrast, in moving water, the entire volume has to. In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze. if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point.
From exotibrjf.blob.core.windows.net
Mechanical Weathering Geography Examples at Carla Chapman blog How Can Water Be Below Freezing And Not Freeze Do you know the freezing point of water? Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. we learn in grade school. How Can Water Be Below Freezing And Not Freeze.
From www.metoffice.gov.uk
Freezing rain Met Office How Can Water Be Below Freezing And Not Freeze the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. Is the freezing point the same as the melting point? then, a thin layer at the surface starts to freeze while floating on the denser water below. Here is a look at the temperature of the freezing point, the factors that affect it,. How Can Water Be Below Freezing And Not Freeze.
From www.youtube.com
Freezer Isn't Freezing — Freezer Troubleshooting YouTube How Can Water Be Below Freezing And Not Freeze Do you know the freezing point of water? if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. Is the freezing point the same as the melting point? then, a thin layer at the surface starts to freeze while floating on the denser water below. the. How Can Water Be Below Freezing And Not Freeze.
From whatsinsight.org
What Temp Does Water Freeze? Science What's Insight How Can Water Be Below Freezing And Not Freeze By contrast, in moving water, the entire volume has to. Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. now, chemists may have solved one enigma by. How Can Water Be Below Freezing And Not Freeze.
From www.slideserve.com
PPT Water, water everywhere! PowerPoint Presentation, free download How Can Water Be Below Freezing And Not Freeze By contrast, in moving water, the entire volume has to. then, a thin layer at the surface starts to freeze while floating on the denser water below. Do you know the freezing point of water? if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. we. How Can Water Be Below Freezing And Not Freeze.
From www.animalia-life.club
Freezing Of Water How Can Water Be Below Freezing And Not Freeze Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. Is the freezing point the same as the melting point? then, a thin. How Can Water Be Below Freezing And Not Freeze.
From www.marshallcountydaily.com
Frozen Pipes Learn how to prevent water pipes from freezing, and how to How Can Water Be Below Freezing And Not Freeze the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. Is the freezing point the same as the melting point? Here is a look at the temperature of the freezing point, the factors that affect. How Can Water Be Below Freezing And Not Freeze.
From purelywholesome.com
How to keep livestock water from freezing Purely Wholesome Farm How Can Water Be Below Freezing And Not Freeze the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. now, chemists. How Can Water Be Below Freezing And Not Freeze.
From www.wtamu.edu
Can water stay liquid below zero degrees Celsius? Science Questions How Can Water Be Below Freezing And Not Freeze we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. then, a thin layer at the surface starts to freeze while floating on the denser water below. the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. In clouds, scientists have found supercooled water droplets as. How Can Water Be Below Freezing And Not Freeze.
From www.thoughtco.com
What Is the Freezing Point of Water? How Can Water Be Below Freezing And Not Freeze we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. Is the freezing point the same as the melting point? if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. Here is a look at the temperature of the freezing point,. How Can Water Be Below Freezing And Not Freeze.
From dxolovtcb.blob.core.windows.net
What To Do When Freezer Isn T Freezing at Joseph Buchanan blog How Can Water Be Below Freezing And Not Freeze the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. then, a thin layer at the surface starts to freeze while floating on the denser water below. By contrast, in moving water, the entire volume has to. Here is a look at the temperature of the freezing point, the factors that affect it,. How Can Water Be Below Freezing And Not Freeze.
From www.youtube.com
Water DOESN'T Freeze At Zero Degrees?! BBC Earth Kids YouTube How Can Water Be Below Freezing And Not Freeze then, a thin layer at the surface starts to freeze while floating on the denser water below. Is the freezing point the same as the melting point? In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze.. How Can Water Be Below Freezing And Not Freeze.
From jicuhurikki.blogspot.com
Can The Ocean Freeze / Why Doesn't The Ocean Freeze Like Rivers And How Can Water Be Below Freezing And Not Freeze In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze. the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. we learn in grade school that water freezes at zero degrees. How Can Water Be Below Freezing And Not Freeze.
From beezzly.com
How Long Does It Take For Water to Freeze? Beezzly How Can Water Be Below Freezing And Not Freeze we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. then, a thin layer at the surface starts to freeze while floating on the denser water below. Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. . How Can Water Be Below Freezing And Not Freeze.
From dxouiuygg.blob.core.windows.net
How To Cool Water Below Freezing at Thomas Pineiro blog How Can Water Be Below Freezing And Not Freeze then, a thin layer at the surface starts to freeze while floating on the denser water below. Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. now, chemists may have solved one enigma by showing how cold water can get before it absolutely. How Can Water Be Below Freezing And Not Freeze.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled How Can Water Be Below Freezing And Not Freeze then, a thin layer at the surface starts to freeze while floating on the denser water below. In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze. By contrast, in moving water, the entire volume has to.. How Can Water Be Below Freezing And Not Freeze.
From www.animalia-life.club
Freezing Of Water How Can Water Be Below Freezing And Not Freeze Is the freezing point the same as the melting point? if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. now, chemists may. How Can Water Be Below Freezing And Not Freeze.
From chescotimes.com
Tips to keep water pipes from freezing How Can Water Be Below Freezing And Not Freeze Do you know the freezing point of water? In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze. now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. . How Can Water Be Below Freezing And Not Freeze.
From www.familyhandyman.com
If You Have Frost in Your Freezer, This is What it Means Family Handyman How Can Water Be Below Freezing And Not Freeze In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze. now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. Is the freezing point the same as the melting. How Can Water Be Below Freezing And Not Freeze.
From delightjar.com
Is It Safe to Freeze Your Water Bottle? Delight Jar How Can Water Be Below Freezing And Not Freeze now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. In clouds,. How Can Water Be Below Freezing And Not Freeze.
From baileylineroad.com
How to Keep Water Intake Lines from Freezing How Can Water Be Below Freezing And Not Freeze now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. . How Can Water Be Below Freezing And Not Freeze.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii How Can Water Be Below Freezing And Not Freeze Do you know the freezing point of water? Is the freezing point the same as the melting point? we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. the freezing point of water. How Can Water Be Below Freezing And Not Freeze.
From www.animalia-life.club
Freezing Of Water How Can Water Be Below Freezing And Not Freeze then, a thin layer at the surface starts to freeze while floating on the denser water below. Do you know the freezing point of water? In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze. Here is. How Can Water Be Below Freezing And Not Freeze.
From www.wcnc.com
Drip faucets in freezing weather to prevent pipe burst How Can Water Be Below Freezing And Not Freeze then, a thin layer at the surface starts to freeze while floating on the denser water below. if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. Is the freezing point the same as the melting point? In clouds, scientists have found supercooled water droplets as chilly. How Can Water Be Below Freezing And Not Freeze.
From www.youtube.com
Why Don’t Oceans Freeze? CURIOSITY YouTube How Can Water Be Below Freezing And Not Freeze if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. Do you. How Can Water Be Below Freezing And Not Freeze.
From www.usatoday.com
How to keep pipes from freezing and fix frozen pipes this winter How Can Water Be Below Freezing And Not Freeze the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. By contrast, in moving water, the entire volume has to. then, a thin layer at the surface starts to freeze while floating on the denser water. How Can Water Be Below Freezing And Not Freeze.
From ar.inspiredpencil.com
Freezing How Can Water Be Below Freezing And Not Freeze Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze. now, chemists may. How Can Water Be Below Freezing And Not Freeze.
From www.slideserve.com
PPT Ch 3 The Polarity of Water and Its Properties PowerPoint How Can Water Be Below Freezing And Not Freeze Do you know the freezing point of water? Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46. How Can Water Be Below Freezing And Not Freeze.
From www.pinterest.com
How to Prevent Your Pipes From Freezing As Temps Drop Frozen pipes How Can Water Be Below Freezing And Not Freeze In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze. we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. the freezing point of water is 32 degrees fahrenheit, 0. How Can Water Be Below Freezing And Not Freeze.
From blograng.com
Can water freeze in 20 minutes? How Can Water Be Below Freezing And Not Freeze Is the freezing point the same as the melting point? now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it. How Can Water Be Below Freezing And Not Freeze.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled How Can Water Be Below Freezing And Not Freeze Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. By contrast, in moving water, the entire volume has to. Do you know the freezing point of water? if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid. How Can Water Be Below Freezing And Not Freeze.
From applianceexpresstx.com
How to Fix Freezer Leaking Water into Fridge Appliance Express How Can Water Be Below Freezing And Not Freeze we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. By contrast, in moving water, the entire volume has to. then, a thin layer at the surface starts to freeze while floating on the denser water below. In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and. How Can Water Be Below Freezing And Not Freeze.
From dxouiuygg.blob.core.windows.net
How To Cool Water Below Freezing at Thomas Pineiro blog How Can Water Be Below Freezing And Not Freeze the freezing point of water is 32 degrees fahrenheit, 0 degrees celsius, and 273.15 kelvin. In clouds, scientists have found supercooled water droplets as chilly as minus 40 c, and in a lab in 2014, they cooled water to a staggering minus 46 c before it froze. we learn in grade school that water freezes at zero degrees. How Can Water Be Below Freezing And Not Freeze.
From knowhowcommunity.org
How Fast Does Water Freeze Know How Community How Can Water Be Below Freezing And Not Freeze if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its normal. Is the freezing point the same as the melting point? we learn in grade school that water freezes at zero degrees celsius, but that’s seldom true. In clouds, scientists have found supercooled water droplets as chilly as. How Can Water Be Below Freezing And Not Freeze.
From www.domeats.com
How Long Does It Take for Water to Freeze? Temperature Explained Dom Eats How Can Water Be Below Freezing And Not Freeze Is the freezing point the same as the melting point? By contrast, in moving water, the entire volume has to. now, chemists may have solved one enigma by showing how cold water can get before it absolutely must. if water is cooled too slowly, it can become supercooled liquid, meaning that it remains in liquid form below its. How Can Water Be Below Freezing And Not Freeze.