Bleaching Powder Reacts With Dilute Hydrochloric Acid . Acids react with metals, bases and carbonates to produce salts. Sodium thiosulfate solution reacts with dilute hydrochloric acid: Neutralisation is the reaction between an acid and a base. Indicators are used to determine if a solution is acidic or alkaline. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we can use stoichiom. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. The chemical formula of bleaching powder is caocl 2.
from www.numerade.com
Indicators are used to determine if a solution is acidic or alkaline. Sodium thiosulfate solution reacts with dilute hydrochloric acid: Neutralisation is the reaction between an acid and a base. The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. Acids react with metals, bases and carbonates to produce salts. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. The chemical formula of bleaching powder is caocl 2. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we can use stoichiom. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown.
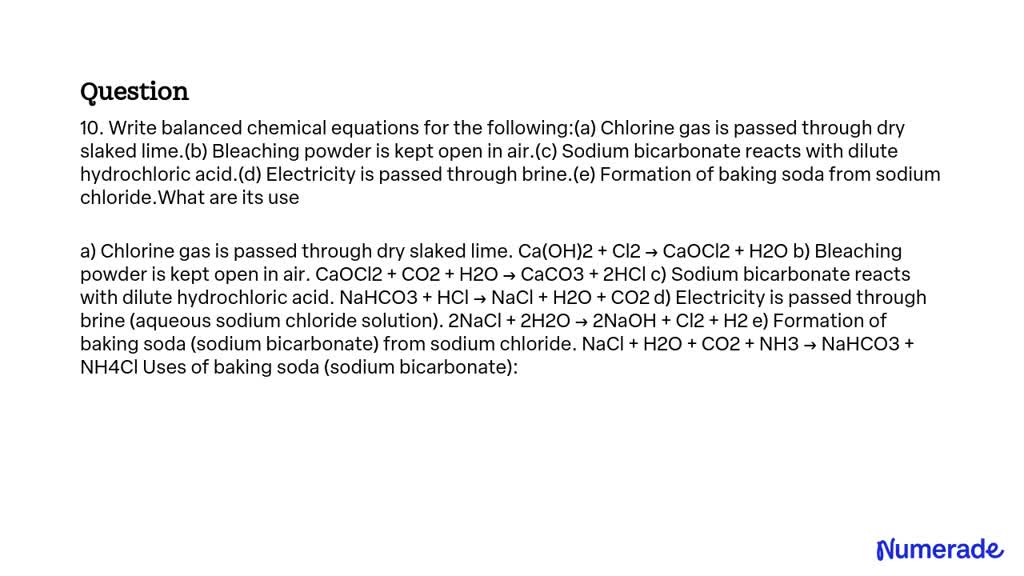
SOLVED 10. Write balanced chemical equations for the following (a
Bleaching Powder Reacts With Dilute Hydrochloric Acid When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Acids react with metals, bases and carbonates to produce salts. Neutralisation is the reaction between an acid and a base. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we can use stoichiom. Indicators are used to determine if a solution is acidic or alkaline. Sodium thiosulfate solution reacts with dilute hydrochloric acid: The chemical formula of bleaching powder is caocl 2. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen.
From brainly.in
What happens when bleaching powder reacts with dilute sulphuric acid Bleaching Powder Reacts With Dilute Hydrochloric Acid When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. The chemical formula of bleaching powder is caocl 2. To calculate the mass of bleaching powder (c a (o c l) 2. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Bleaching Powder Reacts With Dilute Hydrochloric Acid Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Neutralisation is the reaction between an acid and a base. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we can use stoichiom. The chemical formula of bleaching powder. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From quizlet.com
investigate reactions between dilute hydrochloric and sulfuric acids Bleaching Powder Reacts With Dilute Hydrochloric Acid When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Acids react with metals, bases and carbonates to produce salts. The chemical formula of bleaching powder is caocl 2. Indicators are used to determine if a solution is acidic or alkaline. Sodium thiosulfate solution reacts with dilute hydrochloric acid: Sodium thiosulfate + hydrochloric acid. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.toppr.com
. Bleaching powder is obtained by the action of chlorine gas and (a Bleaching Powder Reacts With Dilute Hydrochloric Acid Acids react with metals, bases and carbonates to produce salts. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Sodium thiosulfate solution reacts with dilute hydrochloric acid: When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.youtube.com
Adding baking powder to hydrochloric acid YouTube Bleaching Powder Reacts With Dilute Hydrochloric Acid When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. The chemical formula of bleaching powder is caocl 2. Sodium thiosulfate solution reacts with dilute hydrochloric acid: The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. Indicators are used to determine. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.youtube.com
Bleaching powder reacts with a few drops of conc.`HCl` to yield YouTube Bleaching Powder Reacts With Dilute Hydrochloric Acid Indicators are used to determine if a solution is acidic or alkaline. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Sodium thiosulfate solution reacts with dilute hydrochloric acid: Acids react with metals, bases and carbonates to produce salts. The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.teachoo.com
Metal compound A reacts with dilute hydrochloric acid to produce Bleaching Powder Reacts With Dilute Hydrochloric Acid When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Sodium thiosulfate solution reacts with dilute hydrochloric acid: To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.slideshare.net
Metals Reactivity Series Bleaching Powder Reacts With Dilute Hydrochloric Acid Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Indicators are used to determine if a solution is acidic or alkaline. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Neutralisation is the reaction between an acid and a base. The bleaching powder in the presence of insufficient dilute acids acts. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.aakash.ac.in
Bleaching Powder Formula, Properties and Uses CBSE Chemistry Notes Bleaching Powder Reacts With Dilute Hydrochloric Acid Indicators are used to determine if a solution is acidic or alkaline. Neutralisation is the reaction between an acid and a base. The chemical formula of bleaching powder is caocl 2. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Sodium thiosulfate. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From brainly.in
what happen when dilute hydrochloric acid is added to the following Bleaching Powder Reacts With Dilute Hydrochloric Acid Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we can use stoichiom.. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.alamy.com
calcium carbonate reacts with dilute hydrochloric acid to produce Stock Bleaching Powder Reacts With Dilute Hydrochloric Acid Indicators are used to determine if a solution is acidic or alkaline. Neutralisation is the reaction between an acid and a base. Sodium thiosulfate solution reacts with dilute hydrochloric acid: To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From byjus.com
36 Write the name and formula of cleaning powder. What happens when Bleaching Powder Reacts With Dilute Hydrochloric Acid Neutralisation is the reaction between an acid and a base. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.numerade.com
SOLVED (a) What is bleaching powder? How is bleaching powder prepared Bleaching Powder Reacts With Dilute Hydrochloric Acid Indicators are used to determine if a solution is acidic or alkaline. Neutralisation is the reaction between an acid and a base. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Acids react with metals, bases and carbonates to produce salts. Sodium thiosulfate solution reacts with dilute hydrochloric acid: To calculate the mass of bleaching powder (c a. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From study.com
Sodium Thiosulfate & Hydrochloric Acid Experiment Lesson Bleaching Powder Reacts With Dilute Hydrochloric Acid The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Acids react with metals, bases and carbonates to produce salts. Sodium thiosulfate solution reacts with dilute hydrochloric acid: To calculate the mass of bleaching powder (c a. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.numerade.com
SOLVED 10. Write balanced chemical equations for the following (a Bleaching Powder Reacts With Dilute Hydrochloric Acid The chemical formula of bleaching powder is caocl 2. Indicators are used to determine if a solution is acidic or alkaline. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we can use stoichiom. When dilute hydrochloric acid is added. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From brainly.in
Q10. What happens when dilute HCl is added to the following and Bleaching Powder Reacts With Dilute Hydrochloric Acid Sodium thiosulfate solution reacts with dilute hydrochloric acid: The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. The chemical formula of bleaching powder is caocl 2. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. When dilute hydrochloric acid is added to it, it reacts. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From medicolab.africa
Boric Acid Powder MedicoLab Bleaching Powder Reacts With Dilute Hydrochloric Acid Sodium thiosulfate solution reacts with dilute hydrochloric acid: Neutralisation is the reaction between an acid and a base. Acids react with metals, bases and carbonates to produce salts. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. The bleaching powder in the. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.youtube.com
What Happens when Magnesium reacts with Acetic Acid And Dilute Bleaching Powder Reacts With Dilute Hydrochloric Acid Indicators are used to determine if a solution is acidic or alkaline. The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Neutralisation is the reaction between an acid and a base. Sodium thiosulfate solution reacts with. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.toppr.com
Write balanced chemical equations for the followinga) Calcium Bleaching Powder Reacts With Dilute Hydrochloric Acid The chemical formula of bleaching powder is caocl 2. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Neutralisation is the reaction between an acid and a base. Indicators are used to determine if a solution is acidic or alkaline. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. To calculate. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.youtube.com
Metal compound A reacts with dilute hydrochloric acid to produce Bleaching Powder Reacts With Dilute Hydrochloric Acid Indicators are used to determine if a solution is acidic or alkaline. Sodium thiosulfate solution reacts with dilute hydrochloric acid: The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. Neutralisation is the reaction between an acid and a base. When dilute hydrochloric acid is added to it,. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.chegg.com
Solved 1 Dilute hydrochloric acid reacts with sodium Bleaching Powder Reacts With Dilute Hydrochloric Acid Sodium thiosulfate solution reacts with dilute hydrochloric acid: The chemical formula of bleaching powder is caocl 2. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Acids react with metals, bases and carbonates to produce salts. The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From niasrfrank.blogspot.com
Calcium Reaction With Dilute Hydrochloric Acid NiasrFrank Bleaching Powder Reacts With Dilute Hydrochloric Acid Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Acids react with metals, bases and carbonates to produce salts. Neutralisation is the reaction between an acid and a base. The chemical formula of bleaching powder is caocl 2. Indicators are used to determine if a solution is acidic or alkaline. Sodium thiosulfate solution reacts with dilute hydrochloric acid:. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.youtube.com
Sodium Thiosulphate Reaction With Hydrochloric Acid YouTube Bleaching Powder Reacts With Dilute Hydrochloric Acid The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Acids react with metals, bases and carbonates to produce salts. Neutralisation is the reaction between an acid and a base. Sodium thiosulfate solution reacts with dilute hydrochloric. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.studocu.com
Project 1 STERILIZATION OF WATER BY USING BLEACHING POWDER CHEMISTRY Bleaching Powder Reacts With Dilute Hydrochloric Acid Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. The chemical formula of bleaching powder is caocl 2. The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. Sodium thiosulfate. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.youtube.com
Bleach Vs Hydrochloric Acid How it reacts [Must Watch] YouTube Bleaching Powder Reacts With Dilute Hydrochloric Acid The chemical formula of bleaching powder is caocl 2. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Indicators are used to determine if a solution is acidic or alkaline. Neutralisation is the reaction between an acid and a base. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. Acids react. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.doubtnut.com
On adding dilute hydrochloric acid to copper oxide powder, the solutio Bleaching Powder Reacts With Dilute Hydrochloric Acid Acids react with metals, bases and carbonates to produce salts. Sodium thiosulfate + hydrochloric acid → sodium chloride + water +. The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. Indicators are used to determine if a solution is acidic or alkaline. Neutralisation is the reaction between. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.doubtnut.com
Chemical Tests between Dilute hydrochloric acid and dilute sulphur Bleaching Powder Reacts With Dilute Hydrochloric Acid When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. The chemical formula of bleaching powder is caocl 2. Sodium thiosulfate solution reacts with dilute hydrochloric acid: Neutralisation is the reaction between an acid and a base. Indicators are used to determine if a solution is acidic or alkaline. Acids react with metals, bases. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.chemwifi.com
Bleaching powder chemical formula Bleaching Powder Reacts With Dilute Hydrochloric Acid To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we can use stoichiom. Acids react with metals, bases and carbonates to produce salts. The chemical formula of bleaching powder is caocl 2. The bleaching powder in the presence of insufficient. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.youtube.com
Bleaching powder reacts with dilute acids to produce_____ CLASS 10 Bleaching Powder Reacts With Dilute Hydrochloric Acid The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. Acids react with metals, bases and carbonates to produce salts. Sodium thiosulfate solution reacts with dilute hydrochloric acid: Indicators are used to determine if a solution is acidic or alkaline. Sodium thiosulfate + hydrochloric acid → sodium chloride. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.teachoo.com
Case Base MCQ The reaction between MnO2 with HCl is depicted in Bleaching Powder Reacts With Dilute Hydrochloric Acid When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Indicators are used to determine if a solution is acidic or alkaline. Neutralisation is the reaction between an acid and a base. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.slideshare.net
Metals Bleaching Powder Reacts With Dilute Hydrochloric Acid The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we can use stoichiom. When dilute hydrochloric acid is. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.pinchepreciado.com
Reaction Of Zinc Granules With Dilute Sulphuric Acid And, 59 OFF Bleaching Powder Reacts With Dilute Hydrochloric Acid Neutralisation is the reaction between an acid and a base. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we can use stoichiom. Sodium. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.youtube.com
Q.11 What happens when Dilute Hydrochloric acid is added to iron Bleaching Powder Reacts With Dilute Hydrochloric Acid Neutralisation is the reaction between an acid and a base. The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. When dilute hydrochloric acid is added to it, it reacts to release chlorine gas as shown. Acids react with metals, bases and carbonates to produce salts. Indicators are. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.scribd.com
Magnesium Reacts With Dilute Hydrochloric Acid in a Conical Flask Which Bleaching Powder Reacts With Dilute Hydrochloric Acid The chemical formula of bleaching powder is caocl 2. Neutralisation is the reaction between an acid and a base. To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we can use stoichiom. Indicators are used to determine if a solution. Bleaching Powder Reacts With Dilute Hydrochloric Acid.
From www.doubtnut.com
What happens when Bleaching powder reacts with dilute sulphuric Bleaching Powder Reacts With Dilute Hydrochloric Acid Neutralisation is the reaction between an acid and a base. Indicators are used to determine if a solution is acidic or alkaline. Sodium thiosulfate solution reacts with dilute hydrochloric acid: To calculate the mass of bleaching powder (c a (o c l) 2 ) needed to produce 400 c m 3 of chlorine gas (c l 2 ), ⇒ we. Bleaching Powder Reacts With Dilute Hydrochloric Acid.