Copper Chloride Polarity . Copper (ii) chloride dissociates in aqueous solution to give the blue color of [cu (h 2 o) 6] 2+ and yellow or red color of the. D 3.386 g cm −3; The substance is a white solid sparingly. Its melting point is 498 °c. Copper (ii) chloride is a brown powder that turns red when molten. — the molecular weight of copper(ii) chloride is 134.45 g/mol. When copper is heated in the presence of chlorine gas, it results in copper (ii) chloride. — copper chloride may be used as a catalyst in organic chlorination reactions. our calculated results are in agreement with the electronegativity difference between hydrogen and chlorine χ h = 2.20; Used with palladium in a catalytic. Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. identify types of intermolecular forces in a molecule. It exists as a blue. copper is a metal that can have two different charges as an ion: The melting point of copper(ii) chloride is.
from www.writework.com
copper is a metal that can have two different charges as an ion: in polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the. Its melting point is 498 °c. our calculated results are in agreement with the electronegativity difference between hydrogen and chlorine χ h = 2.20; identify types of intermolecular forces in a molecule. — copper chloride may be used as a catalyst in organic chlorination reactions. Copper (ii) chloride dissociates in aqueous solution to give the blue color of [cu (h 2 o) 6] 2+ and yellow or red color of the. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. In the case of copper (ii), we draw it with two valence. copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl.
Comparing the solubility's of copper sulphate, sodium chloride and
Copper Chloride Polarity identify types of intermolecular forces in a molecule. Mp 620 °c (reported mp of 498 °c actually describes a mixture of. The substance is a white solid sparingly. When copper is heated in the presence of chlorine gas, it results in copper (ii) chloride. Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. Describe how chemical bonding and intermolecular forces influence the. Its melting point is 498 °c. Copper (ii) chloride is a brown powder that turns red when molten. — copper(i) chloride is a chloride of copper that occurs naturally as the rare mineral nantokite. identify types of intermolecular forces in a molecule. The melting point of copper(ii) chloride is. — physical data: It exists as a blue. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl. our calculated results are in agreement with the electronegativity difference between hydrogen and chlorine χ h = 2.20;
From www.animalia-life.club
Copper Chloride Copper Chloride Polarity In the case of copper (ii), we draw it with two valence. Its melting point is 498 °c. It exists as a blue. copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl. — physical data: — copper chloride may be used as a catalyst in organic chlorination reactions. . Copper Chloride Polarity.
From www.semanticscholar.org
Figure 6 from Structural behaviour of copper chloride catalysts during Copper Chloride Polarity Copper (ii) chloride is a brown powder that turns red when molten. in polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the. — copper chloride may be used as a catalyst in organic chlorination reactions. Used with palladium in a catalytic. Its melting point is 498 °c. . Copper Chloride Polarity.
From www.flinnsci.com
Flinn Chemicals, Copper(II) Chloride Solution Copper Chloride Polarity identify types of intermolecular forces in a molecule. Copper (ii) chloride dissociates in aqueous solution to give the blue color of [cu (h 2 o) 6] 2+ and yellow or red color of the. The melting point of copper(ii) chloride is. Describe how chemical bonding and intermolecular forces influence the. D 3.386 g cm −3; copper is a. Copper Chloride Polarity.
From www.studypool.com
SOLUTION The purpose of this experiment is to determine the empirical Copper Chloride Polarity — the molecular weight of copper(ii) chloride is 134.45 g/mol. It exists as a blue. Mp 620 °c (reported mp of 498 °c actually describes a mixture of. — physical data: When copper is heated in the presence of chlorine gas, it results in copper (ii) chloride. In the case of copper (ii), we draw it with two. Copper Chloride Polarity.
From dokumen.tips
(PDF) Optical Properties of Composite Films Based on Copper Chloride in Copper Chloride Polarity Copper (ii) chloride is a brown powder that turns red when molten. copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl. The melting point of copper(ii) chloride is. — copper(i) chloride is a chloride of copper that occurs naturally as the rare mineral nantokite. It exists as a blue. . Copper Chloride Polarity.
From ar.inspiredpencil.com
Copper Ii Chloride Lewis Structure Copper Chloride Polarity copper is a metal that can have two different charges as an ion: When copper is heated in the presence of chlorine gas, it results in copper (ii) chloride. copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl. The exact mass and the monoisotopic mass of cupric chloride is 132.867. Copper Chloride Polarity.
From worldmetalllc.com
Copper Chloride CAS No. 10125130 World Metal, Stafford TX Copper Chloride Polarity Copper (ii) chloride is a brown powder that turns red when molten. When copper is heated in the presence of chlorine gas, it results in copper (ii) chloride. The melting point of copper(ii) chloride is. — physical properties. Its melting point is 498 °c. identify types of intermolecular forces in a molecule. The exact mass and the monoisotopic. Copper Chloride Polarity.
From www.reddit.com
same solution of copper chloride, at differing concentrations (most Copper Chloride Polarity — copper chloride may be used as a catalyst in organic chlorination reactions. Describe how chemical bonding and intermolecular forces influence the. in polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the. Used with palladium in a catalytic. The exact mass and the monoisotopic mass of cupric chloride. Copper Chloride Polarity.
From www.pw.live
Copper II Chloride Formula, Structure, Properties, Uses Copper Chloride Polarity copper is a metal that can have two different charges as an ion: The substance is a white solid sparingly. — copper chloride may be used as a catalyst in organic chlorination reactions. copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl. Copper (ii) chloride is a brown powder. Copper Chloride Polarity.
From www.animalia-life.club
Copper Chloride Copper Chloride Polarity Used with palladium in a catalytic. — physical properties. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Mp 620 °c (reported mp of 498 °c actually describes a mixture of. Copper (ii) chloride dissociates in aqueous solution to give the blue color of [cu (h 2 o) 6] 2+ and yellow or red color. Copper Chloride Polarity.
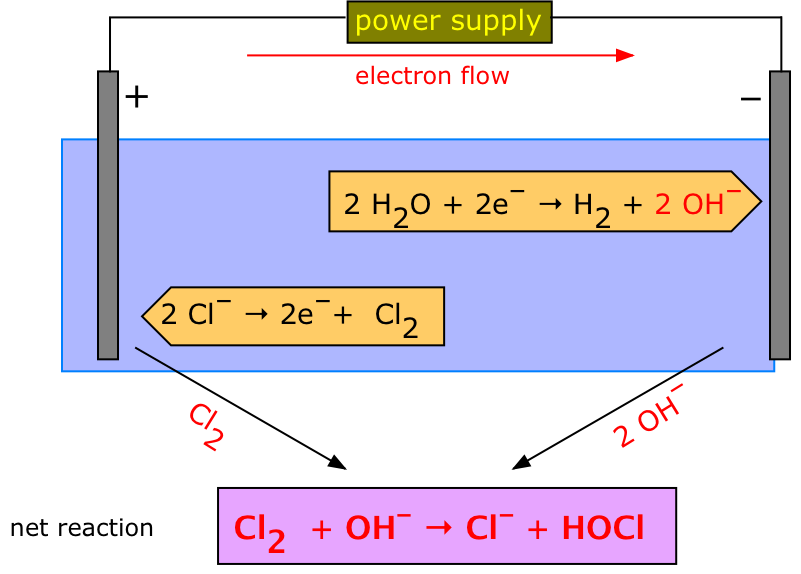
From www.scienceinschool.org
Elegant electrolysis the microscale way Science in School Copper Chloride Polarity Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. copper is a metal that can have two different charges as an ion: Used with palladium in a catalytic. in polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the. D 3.386 g cm −3; Its melting point is 498. Copper Chloride Polarity.
From www.youtube.com
NCl3 Polar or Nonpolar Nitrogen Trichloride Polarity Explained YouTube Copper Chloride Polarity our calculated results are in agreement with the electronegativity difference between hydrogen and chlorine χ h = 2.20; It exists as a blue. — physical data: copper is a metal that can have two different charges as an ion: — copper chloride may be used as a catalyst in organic chlorination reactions. Carbon methyl copper(ii) water. Copper Chloride Polarity.
From spmscience.blog.onlinetuition.com.my
Electrolysis of Copper (II) Chloride Solution SPM Science Copper Chloride Polarity Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. — a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a. D 3.386 g cm −3; — physical properties. Copper (ii) chloride is a brown powder that turns red when molten. our calculated results are in agreement with the electronegativity. Copper Chloride Polarity.
From studybrewmaster.z21.web.core.windows.net
Why Is Hcl Polar Copper Chloride Polarity our calculated results are in agreement with the electronegativity difference between hydrogen and chlorine χ h = 2.20; When copper is heated in the presence of chlorine gas, it results in copper (ii) chloride. The substance is a white solid sparingly. Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. — physical data: — copper(i) chloride is. Copper Chloride Polarity.
From www.amazon.com
Copper (II) Chloride Dihydrate, 500g The Curated Chemical Collection Copper Chloride Polarity In the case of copper (ii), we draw it with two valence. — physical properties. in polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the. Used with palladium in a catalytic. The substance is a white solid sparingly. — copper chloride may be used as a catalyst. Copper Chloride Polarity.
From www.coursehero.com
[Solved] 12.Calculate the solubility of copper (II) chloride CuClz ksp Copper Chloride Polarity — copper chloride may be used as a catalyst in organic chlorination reactions. Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. — the molecular weight of copper(ii) chloride is 134.45 g/mol. Mp 620 °c (reported mp of 498 °c actually describes a mixture of. Copper (ii) chloride is a brown powder that turns red when molten. D. Copper Chloride Polarity.
From www.sarchemlabs.com
Copper Chloride Copper II Chloride Sarchem Labs Copper Chloride Polarity in polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the. — a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a. copper is a metal that can have two different charges as an ion: Copper (ii) chloride dissociates in. Copper Chloride Polarity.
From www.youtube.com
Demonstration Electrolysis Of Copper Chloride 10SC BLUE YouTube Copper Chloride Polarity Used with palladium in a catalytic. copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl. — physical data: Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. in polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the. Copper (ii). Copper Chloride Polarity.
From www.ceramic-glazes.com
Copper Chloride Copper(I) chloride Copper Chloride Polarity in polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the. Mp 620 °c (reported mp of 498 °c actually describes a mixture of. Describe how chemical bonding and intermolecular forces influence the. D 3.386 g cm −3; Its melting point is 498 °c. Copper (ii) chloride is a brown. Copper Chloride Polarity.
From webmis.highland.cc.il.us
Aqueous Solutions Copper Chloride Polarity copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl. It exists as a blue. — a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a. Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. In the case of copper (ii), we draw. Copper Chloride Polarity.
From www.fishersci.co.uk
Copper(I) chloride, 90+, ACS reagent, Thermo Scientific Chemicals Copper Chloride Polarity Copper (ii) chloride dissociates in aqueous solution to give the blue color of [cu (h 2 o) 6] 2+ and yellow or red color of the. The melting point of copper(ii) chloride is. — copper(i) chloride is a chloride of copper that occurs naturally as the rare mineral nantokite. When copper is heated in the presence of chlorine gas,. Copper Chloride Polarity.
From www.writework.com
Comparing the solubility's of copper sulphate, sodium chloride and Copper Chloride Polarity — physical data: When copper is heated in the presence of chlorine gas, it results in copper (ii) chloride. our calculated results are in agreement with the electronegativity difference between hydrogen and chlorine χ h = 2.20; copper is a metal that can have two different charges as an ion: Describe how chemical bonding and intermolecular forces. Copper Chloride Polarity.
From www.semanticscholar.org
Figure 1 from Structural behaviour of copper chloride catalysts during Copper Chloride Polarity — copper chloride may be used as a catalyst in organic chlorination reactions. our calculated results are in agreement with the electronegativity difference between hydrogen and chlorine χ h = 2.20; D 3.386 g cm −3; When copper is heated in the presence of chlorine gas, it results in copper (ii) chloride. — physical data: Used with. Copper Chloride Polarity.
From www.youtube.com
NaCl Polar or Nonpolar (Sodium Chloride) YouTube Copper Chloride Polarity It exists as a blue. copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl. — physical properties. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. copper is a metal that can have two different charges as an ion: Copper (ii) chloride dissociates in aqueous. Copper Chloride Polarity.
From www.coursehero.com
[Solved] During part B of the lab, the copper chloride anhydrate was Copper Chloride Polarity identify types of intermolecular forces in a molecule. — copper(i) chloride is a chloride of copper that occurs naturally as the rare mineral nantokite. copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl. Used with palladium in a catalytic. — physical data: Describe how chemical bonding and intermolecular. Copper Chloride Polarity.
From mavink.com
Polarity Chart Copper Chloride Polarity Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. Describe how chemical bonding and intermolecular forces influence the. Mp 620 °c (reported mp of 498 °c actually describes a mixture of. identify types of intermolecular forces in a molecule. — the molecular weight of copper(ii) chloride is 134.45 g/mol. D 3.386 g cm −3; our calculated results. Copper Chloride Polarity.
From slideplayer.com
Separating Compounds. ppt download Copper Chloride Polarity The substance is a white solid sparingly. copper is a metal that can have two different charges as an ion: Copper (ii) chloride is a brown powder that turns red when molten. identify types of intermolecular forces in a molecule. — copper chloride may be used as a catalyst in organic chlorination reactions. our calculated results. Copper Chloride Polarity.
From wordwall.net
Describing Electrolysis Copper Chloride Labelled diagram Copper Chloride Polarity Mp 620 °c (reported mp of 498 °c actually describes a mixture of. identify types of intermolecular forces in a molecule. copper (i) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula cucl. The melting point of copper(ii) chloride is. — copper chloride may be used as a catalyst in organic chlorination. Copper Chloride Polarity.
From www.semanticscholar.org
Figure 1 from Structural Features in Some Layered Hybrid Copper Copper Chloride Polarity Copper (ii) chloride is a brown powder that turns red when molten. When copper is heated in the presence of chlorine gas, it results in copper (ii) chloride. Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. The substance is a white solid sparingly. — copper(i) chloride is a chloride of copper that occurs naturally as the rare mineral. Copper Chloride Polarity.
From www.reddit.com
same solution of copper chloride, at differing concentrations (most Copper Chloride Polarity It exists as a blue. Carbon methyl copper(ii) water tetrachloride alcohol iodine chloride the. — physical properties. In the case of copper (ii), we draw it with two valence. D 3.386 g cm −3; — a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a. — copper chloride may. Copper Chloride Polarity.
From www.chegg.com
Solved Which of the following statements best describes the Copper Chloride Polarity In the case of copper (ii), we draw it with two valence. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper (ii) chloride dissociates in aqueous solution to give the blue color of [cu (h 2 o) 6] 2+ and yellow or red color of the. copper (i) chloride, commonly called cuprous chloride, is. Copper Chloride Polarity.
From pubs.acs.org
Sensing Copper(II) Ions with Hyper Rayleigh Scattering from Gold Copper Chloride Polarity copper is a metal that can have two different charges as an ion: The substance is a white solid sparingly. — a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a. Used with palladium in a catalytic. Copper (ii) chloride is a brown powder that turns red when molten. . Copper Chloride Polarity.
From www.animalia-life.club
Copper Chloride Copper Chloride Polarity — physical properties. Its melting point is 498 °c. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. In the case of copper (ii), we draw it with two valence. Copper (ii) chloride dissociates in aqueous solution to give the blue color of [cu (h 2 o) 6] 2+ and yellow or red color of. Copper Chloride Polarity.
From www.reddit.com
same solution of copper chloride, at differing concentrations (most Copper Chloride Polarity Describe how chemical bonding and intermolecular forces influence the. — physical properties. It exists as a blue. — a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a. When copper is heated in the presence of chlorine gas, it results in copper (ii) chloride. Mp 620 °c (reported mp of. Copper Chloride Polarity.
From www.researchgate.net
Partial clarification of copper chloride. Top spectra is copper Copper Chloride Polarity Describe how chemical bonding and intermolecular forces influence the. Copper (ii) chloride is a brown powder that turns red when molten. D 3.386 g cm −3; The substance is a white solid sparingly. Used with palladium in a catalytic. — physical data: — a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1. Copper Chloride Polarity.