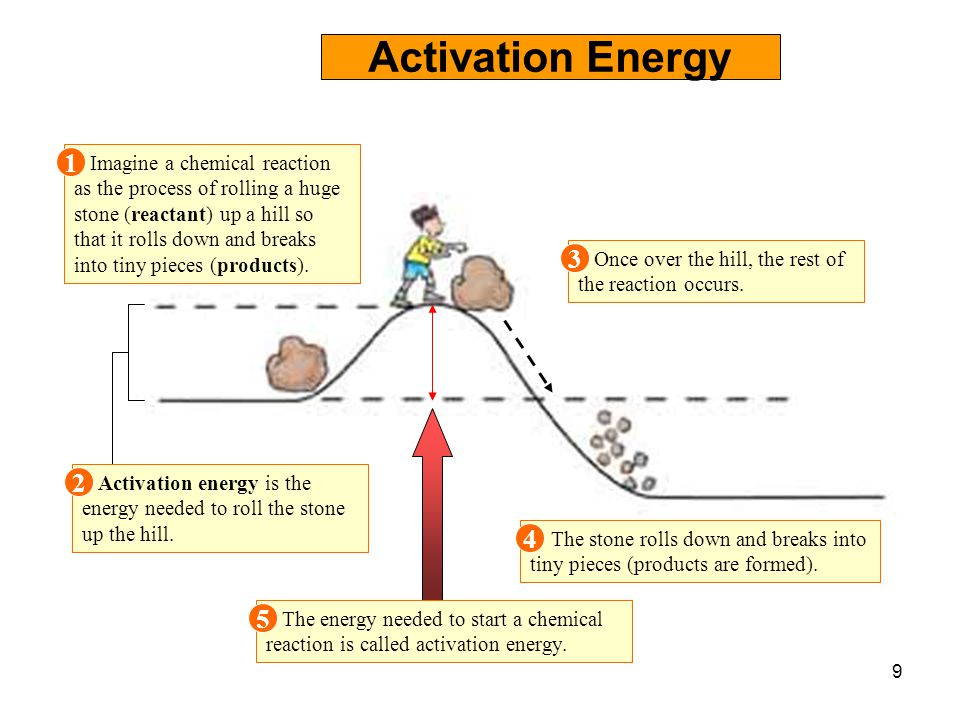
Threshold Energy Is Also Called . the minimal amount of energy needed for a particle to pass through a wall or potential energy well is known as the threshold. the threshold energy is the minimum energy that a particle must have in order to undergo a specific reaction. in order to effectively initiate a reaction, collisions must be sufficiently energetic (kinetic energy) to break chemical bonds;. For a particular reaction, the threshold energy might be as shown here: The minimum energy that all colliding molecules must possess in order to make the collisions effective and. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. what is the threshold energy?. The threshold energy is always greater than or equal to, the rest energy of the resulting particle. what is threshold energy. this energy threshold, called the activation energy, was first postulated in 1888 by the swedish chemist svante. the difference between threshold energy and average energy of reactant molecules is called: the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the. in chemistry, activation energy, also called threshold energy, is a term introduced in 1889 by svante arrhenius that is defined. Threshold energy = energy of. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles.
from www.vrogue.co
revision notes on 2.4.2 threshold frequency & work function for the aqa a level physics syllabus, written by the physics experts at save my exams. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. the threshold energy is the energy that these molecules must have in order for a reaction to take place. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. the difference between threshold energy and average energy of reactant molecules is called: in chemistry, activation energy, also called threshold energy, is a term introduced in 1889 by svante arrhenius that is defined. It is the minimum amount of energy which the reactant molecules must possess for the. there is a threshold frequency below which no electrons are ejected, because the individual photon interacting. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. The minimum kinetic energy that a molecule must possess in order to bring about an.
What The Significance Of “er” In The Diagram A Av vrogue.co
Threshold Energy Is Also Called in chemistry, activation energy, also called threshold energy, is a term introduced in 1889 by svante arrhenius that is defined. the difference between threshold energy and average energy of reactant molecules is called: threshold energy is the minimum amount of energy needed for particles to react. The minimum kinetic energy that a molecule must possess in order to bring about an. what is threshold energy. revision notes on 2.4.2 threshold frequency & work function for the aqa a level physics syllabus, written by the physics experts at save my exams. in chemistry, activation energy, also called threshold energy, is a term introduced in 1889 by svante arrhenius that is defined. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. the threshold energy is the minimum energy that a particle must have in order to undergo a specific reaction. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. Initiation energy is characterized as the base measure of additional energy. there is a threshold frequency below which no electrons are ejected, because the individual photon interacting. this energy threshold, called the activation energy, was first postulated in 1888 by the swedish chemist svante. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. Threshold energy = energy of. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two.
From hopetaft.blogspot.com
Hopetaft Beach Ball Energy Threshold Energy Is Also Called what is threshold energy. the threshold energy is the minimum energy that a particle must have in order to undergo a specific reaction. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. there is a threshold frequency below which no electrons are ejected, because the individual photon interacting. . Threshold Energy Is Also Called.
From dxorgzvns.blob.core.windows.net
Threshold Energy Of Nuclear Reaction at Lloyd Mueller blog Threshold Energy Is Also Called there is a threshold frequency below which no electrons are ejected, because the individual photon interacting. Threshold energy = energy of. Threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear decay or fusion. in chemistry, activation energy, also called threshold energy, is a term introduced in. Threshold Energy Is Also Called.
From www.researchgate.net
Variations of remaining energy and threshold energy against prediction Threshold Energy Is Also Called the minimal amount of energy needed for a particle to pass through a wall or potential energy well is known as the threshold. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. what is the threshold energy?. threshold energy is the minimum kinetic energy. Threshold Energy Is Also Called.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Is Also Called what is threshold energy. For a particular reaction, the threshold energy might be as shown here: this energy threshold, called the activation energy, was first postulated in 1888 by the swedish chemist svante. the threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy is the minimum. Threshold Energy Is Also Called.
From www.researchgate.net
(colour online) (a) Calculated threshold energy fluence and (b Threshold Energy Is Also Called threshold energy is the minimum amount of energy needed for particles to react. in chemistry, activation energy, also called threshold energy, is a term introduced in 1889 by svante arrhenius that is defined. It is the minimum amount of energy which the reactant molecules must possess for the. revision notes on 2.4.2 threshold frequency & work function. Threshold Energy Is Also Called.
From byjus.com
26. What is difference between threshold energy and activation energy? Threshold Energy Is Also Called activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. Threshold energy = energy of. in chemistry, activation energy, also called threshold energy, is a term introduced in 1889 by svante arrhenius that is defined. the minimum energy that molecules need to have in order for a reaction. Threshold Energy Is Also Called.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy Is Also Called the threshold energy is the minimum energy that a particle must have in order to undergo a specific reaction. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. the minimal amount of energy needed for a particle to pass through a wall or potential energy well is known. Threshold Energy Is Also Called.
From www.researchgate.net
The threshold energy of the СННН process versus temperature for the Threshold Energy Is Also Called The minimum kinetic energy that a molecule must possess in order to bring about an. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. Threshold energy = energy of. For a particular reaction, the threshold energy might be as shown here: in chemistry, activation energy, also called threshold energy, is a. Threshold Energy Is Also Called.
From www.doubtnut.com
The Threshold energy is given as E(0) and radiation of energy E Threshold Energy Is Also Called The minimum kinetic energy that a molecule must possess in order to bring about an. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. the threshold energy is the minimum energy that a particle must have in order to undergo a specific reaction. threshold energy. Threshold Energy Is Also Called.
From www.diplomageeks.com
Define Threshold frequency,Threshold wavelength,Work function, Stopping Threshold Energy Is Also Called Chemical kinetics board:icse you can ask any doubt from. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. threshold energy is the minimum amount of energy needed for particles to react. there is a threshold frequency below which no electrons are ejected, because the individual. Threshold Energy Is Also Called.
From byjus.com
What is threshold frequency and threshold energy? Threshold Energy Is Also Called the threshold energy is the minimum energy that a particle must have in order to undergo a specific reaction. threshold energy is the minimum amount of energy needed for particles to react. the difference between threshold energy and average energy of reactant molecules is called: what is threshold energy. For a particular reaction, the threshold energy. Threshold Energy Is Also Called.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Threshold Energy Is Also Called The threshold energy is always greater than or equal to, the rest energy of the resulting particle. there is a threshold frequency below which no electrons are ejected, because the individual photon interacting. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the. this energy threshold,. Threshold Energy Is Also Called.
From kunduz.com
[ANSWERED] What is the threshold energy of reaction RP in represented Threshold Energy Is Also Called threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. the minimal amount of energy needed for a particle to pass through a wall or potential energy well is known as the threshold. the minimum amount of energy required to remove an electron from the metal is called the. Threshold Energy Is Also Called.
From www.nuclear-power.com
Critical Energy Threshold Energy for Fission Threshold Energy Is Also Called Threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear decay or fusion. in chemistry, activation energy, also called threshold energy, is a term introduced in 1889 by svante arrhenius that is defined. the threshold energy is the minimum energy that a particle must have in order. Threshold Energy Is Also Called.
From www.researchgate.net
(PDF) Threshold Energy Threshold Energy Is Also Called Initiation energy is characterized as the base measure of additional energy. the difference between threshold energy and average energy of reactant molecules is called: The minimum energy that all colliding molecules must possess in order to make the collisions effective and. the minimal amount of energy needed for a particle to pass through a wall or potential energy. Threshold Energy Is Also Called.
From www.researchgate.net
(a) Energy (Er in a.u.) and the threshold energy (E2s in a.u.) vs. 1 Z Threshold Energy Is Also Called there is a threshold frequency below which no electrons are ejected, because the individual photon interacting. this energy threshold, called the activation energy, was first postulated in 1888 by the swedish chemist svante. revision notes on 2.4.2 threshold frequency & work function for the aqa a level physics syllabus, written by the physics experts at save my. Threshold Energy Is Also Called.
From www.researchgate.net
(a), (b) Threshold energy and connectivity strength distributions for Threshold Energy Is Also Called The threshold energy is always greater than or equal to, the rest energy of the resulting particle. the difference between threshold energy and average energy of reactant molecules is called: what is threshold energy. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. threshold energy is. Threshold Energy Is Also Called.
From www.slideserve.com
PPT Radioactivity 29.3 PowerPoint Presentation ID2979284 Threshold Energy Is Also Called the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. It is the minimum amount of energy which the reactant molecules must possess for the. Chemical kinetics board:icse you can ask any doubt from. in chemistry, activation energy, also called threshold energy, is a term introduced in. Threshold Energy Is Also Called.
From www.researchgate.net
(a) As in figure 4(a) but far from the threshold energy region (ω/I Threshold Energy Is Also Called Initiation energy is characterized as the base measure of additional energy. Threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear decay or fusion. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. the minimum. Threshold Energy Is Also Called.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Is Also Called Initiation energy is characterized as the base measure of additional energy. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. Threshold energy = energy of. Chemical kinetics board:icse you can ask any doubt from. there is a threshold frequency below which no electrons are ejected, because. Threshold Energy Is Also Called.
From exogobhlj.blob.core.windows.net
Threshold Energy For Proton at Irene Browning blog Threshold Energy Is Also Called activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. in chemistry, activation energy, also called threshold energy, is a term introduced in 1889 by svante arrhenius that is defined. the threshold energy is the energy that these molecules must have in order for a reaction to take. Threshold Energy Is Also Called.
From www.youtube.com
What is activation energy Threshold energy energy barrier rate of Threshold Energy Is Also Called Threshold energy = energy of. the threshold energy is the minimum energy that a particle must have in order to undergo a specific reaction. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. revision notes on 2.4.2 threshold frequency & work function for the aqa. Threshold Energy Is Also Called.
From www.numerade.com
SOLVEDDetermine the Q value and the threshold energy for 8^16 O+ 0^1 Threshold Energy Is Also Called what is the threshold energy?. there is a threshold frequency below which no electrons are ejected, because the individual photon interacting. Initiation energy is characterized as the base measure of additional energy. Threshold energy = energy of. the difference between threshold energy and average energy of reactant molecules is called: revision notes on 2.4.2 threshold frequency. Threshold Energy Is Also Called.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Threshold Energy Is Also Called the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the. the difference between threshold energy and average energy of reactant molecules is called: For a particular reaction, the threshold energy might be as shown here: the minimum energy that molecules need to have in order for. Threshold Energy Is Also Called.
From edurev.in
Difference between activation energy and threshold energy I know the Threshold Energy Is Also Called the threshold energy is the energy that these molecules must have in order for a reaction to take place. in order to effectively initiate a reaction, collisions must be sufficiently energetic (kinetic energy) to break chemical bonds;. what is threshold energy. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold. Threshold Energy Is Also Called.
From www.slideserve.com
PPT NE 301 Introduction to Nuclear Science Spring 2012 PowerPoint Threshold Energy Is Also Called in chemistry, activation energy, also called threshold energy, is a term introduced in 1889 by svante arrhenius that is defined. Initiation energy is characterized as the base measure of additional energy. Threshold energy = energy of. threshold energy is the minimum amount of energy needed for particles to react. the minimum amount of energy required to remove. Threshold Energy Is Also Called.
From www.researchgate.net
The threshold energy, √ s th , for K + Λ and K + Σ Download Threshold Energy Is Also Called threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. this energy threshold, called the activation energy, was first postulated in 1888 by the swedish chemist svante. the minimal amount of energy needed for a particle to pass through a wall or potential energy well is known as the. Threshold Energy Is Also Called.
From www.youtube.com
Explain the terms Threshold energy 12 CHEMICAL Threshold Energy Is Also Called threshold energy is the minimum amount of energy needed for particles to react. the threshold energy is the energy that these molecules must have in order for a reaction to take place. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. in chemistry, activation energy, also called. Threshold Energy Is Also Called.
From www.researchgate.net
The threshold energy E 0 is determined by the equation (3.1). The red Threshold Energy Is Also Called the threshold energy is the minimum energy that a particle must have in order to undergo a specific reaction. Threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear decay or fusion. the minimum amount of energy required to remove an electron from the metal is called. Threshold Energy Is Also Called.
From www.chegg.com
Solved Vo Threshold Voltage What is threshold voltage Threshold Energy Is Also Called Threshold energy = energy of. the threshold energy is the energy that these molecules must have in order for a reaction to take place. It is the minimum amount of energy which the reactant molecules must possess for the. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. . Threshold Energy Is Also Called.
From www.researchgate.net
All kinds of threshold energy of pairbreaking excitation in different Threshold Energy Is Also Called For a particular reaction, the threshold energy might be as shown here: the threshold energy is the energy that these molecules must have in order for a reaction to take place. Chemical kinetics board:icse you can ask any doubt from. Initiation energy is characterized as the base measure of additional energy. The minimum energy that all colliding molecules must. Threshold Energy Is Also Called.
From www.youtube.com
Threshold energy required by Reactant particles to form products YouTube Threshold Energy Is Also Called there is a threshold frequency below which no electrons are ejected, because the individual photon interacting. what is threshold energy. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. threshold energy is the minimum amount of energy needed for particles to react. Threshold energy. Threshold Energy Is Also Called.
From www.vrogue.co
What The Significance Of “er” In The Diagram A Av vrogue.co Threshold Energy Is Also Called there is a threshold frequency below which no electrons are ejected, because the individual photon interacting. the threshold energy is the minimum energy that a particle must have in order to undergo a specific reaction. The minimum kinetic energy that a molecule must possess in order to bring about an. this energy threshold, called the activation energy,. Threshold Energy Is Also Called.
From byjus.com
select the incorrect statement (1) The minimum amount of energy Threshold Energy Is Also Called threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. Chemical kinetics board:icse you can ask any doubt from. threshold energy is the minimum amount of energy needed for particles to react. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. . Threshold Energy Is Also Called.
From www.researchgate.net
Relationship between threshold energy and delay of UV and IR pulses Threshold Energy Is Also Called in chemistry, activation energy, also called threshold energy, is a term introduced in 1889 by svante arrhenius that is defined. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. the minimal amount of energy needed for a particle to pass through a wall or potential energy well. Threshold Energy Is Also Called.