Medical Device Distributor Regulatory Requirements Uk . This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. Under those regulations, it’s possible, now,. The medical device regulations have significantly increased the requirements for distributors. You need to understand these requirements. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients.
from www.mantrasystems.co.uk
The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. Under those regulations, it’s possible, now,. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. You need to understand these requirements. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. The medical device regulations have significantly increased the requirements for distributors. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra.
Achieve EU MDR medical device compliance Free Guide 2024
Medical Device Distributor Regulatory Requirements Uk You need to understand these requirements. You need to understand these requirements. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. The medical device regulations have significantly increased the requirements for distributors. Under those regulations, it’s possible, now,.
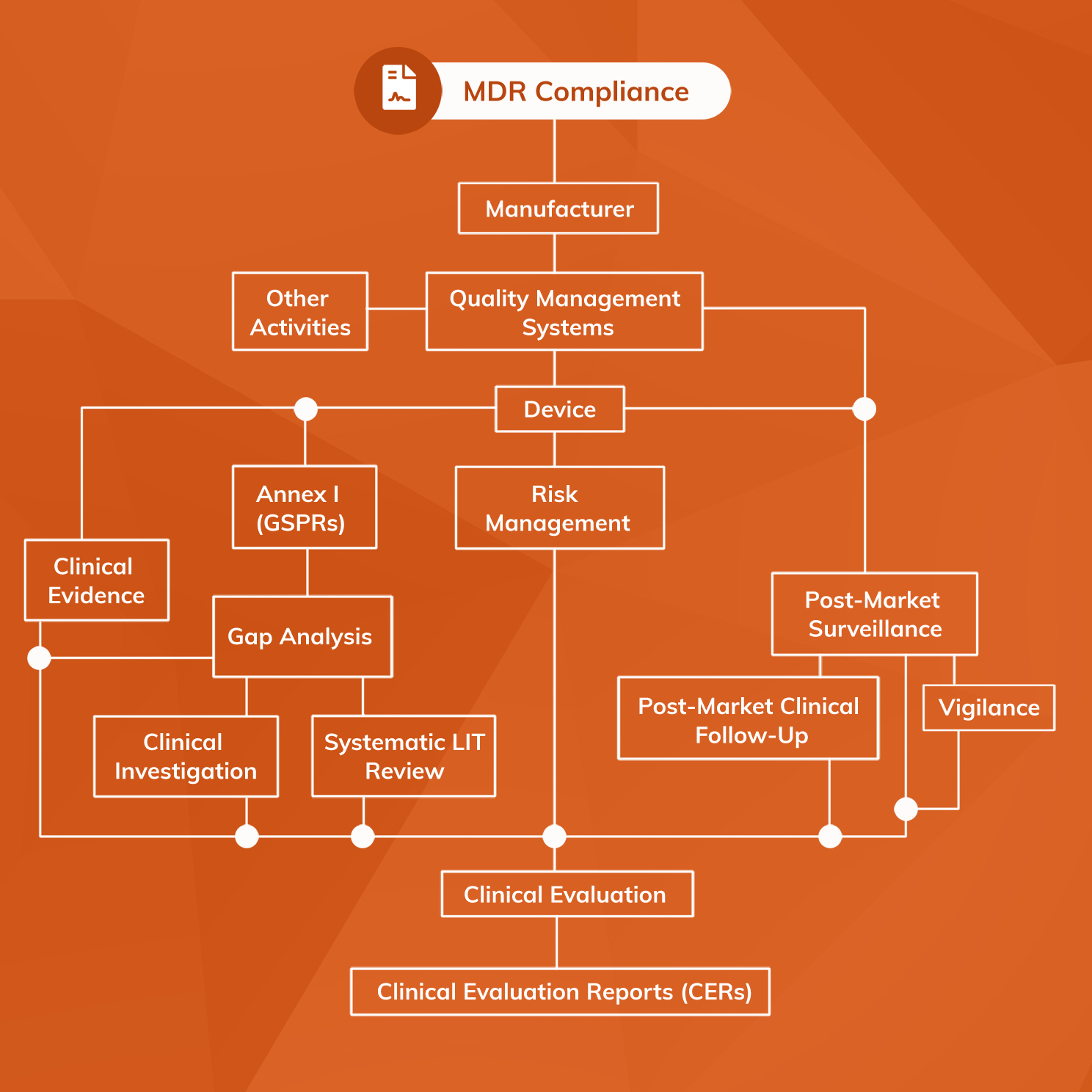
From www.capgemini.com
Remediation implications for medical device manufacturers in changing Medical Device Distributor Regulatory Requirements Uk The medical device regulations have significantly increased the requirements for distributors. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. Under those regulations,. Medical Device Distributor Regulatory Requirements Uk.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Medical Device Distributor Regulatory Requirements Uk You need to understand these requirements. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. The medical device regulations have significantly increased the requirements for distributors. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care. Medical Device Distributor Regulatory Requirements Uk.
From www.argosmultilingual.com
Regulatory Language Requirements for Medical Devices in the EU Medical Device Distributor Regulatory Requirements Uk While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. Under those regulations, it’s possible, now,. The udi system provides. Medical Device Distributor Regulatory Requirements Uk.
From www.presentationeze.com
FDA Regulatory Requirements Medical Devices.PresentationEZE Medical Device Distributor Regulatory Requirements Uk The medical device regulations have significantly increased the requirements for distributors. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an. Medical Device Distributor Regulatory Requirements Uk.
From medicaldevices.freyrsolutions.com
UKCA Marking Requirements for Medical Devices Freyr Medical Devices Medical Device Distributor Regulatory Requirements Uk Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. This guidance sets out what is required to place a. Medical Device Distributor Regulatory Requirements Uk.
From www.heartlungcirc.org
Regulatory Requirements For Medical Devices And Vascular Ageing An Medical Device Distributor Regulatory Requirements Uk The medical device regulations have significantly increased the requirements for distributors. Under those regulations, it’s possible, now,. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. Check the legal requirements you need to meet before you can place a medical device. Medical Device Distributor Regulatory Requirements Uk.
From security.cybellum.com
Intro to Medical Device Standards & Regulations Cybellum Medical Device Distributor Regulatory Requirements Uk You need to understand these requirements. The medical device regulations have significantly increased the requirements for distributors. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. Check the legal requirements you need to meet before you can place a medical device. Medical Device Distributor Regulatory Requirements Uk.
From apacmed.org
Medical Device Regulation Importance and Examples in APAC Medical Device Distributor Regulatory Requirements Uk While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. The medical device regulations have significantly increased the requirements for. Medical Device Distributor Regulatory Requirements Uk.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 Medical Device Distributor Regulatory Requirements Uk The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. The medical device regulations have significantly increased the requirements for distributors. While previously. Medical Device Distributor Regulatory Requirements Uk.
From data1.skinnyms.com
Regulatory Strategy Template For Medical Devices Medical Device Distributor Regulatory Requirements Uk Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. The udi system provides a consistent and standard way to identify medical devices throughout their. Medical Device Distributor Regulatory Requirements Uk.
From www.protoexpress.com
Medical Device Regulations for PCBA Sierra Circuits Medical Device Distributor Regulatory Requirements Uk You need to understand these requirements. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. Check the. Medical Device Distributor Regulatory Requirements Uk.
From www.auxergo.com
The Interactive Guide Under The New EU Regulations on Medical Devices Medical Device Distributor Regulatory Requirements Uk The medical device regulations have significantly increased the requirements for distributors. Under those regulations, it’s possible, now,. You need to understand these requirements. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. The udi system provides a consistent and standard way to identify medical devices throughout. Medical Device Distributor Regulatory Requirements Uk.
From crfweb.com
Medical Device Regulations Medical Device Distributor Regulatory Requirements Uk You need to understand these requirements. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. The udi system provides a consistent and standard way. Medical Device Distributor Regulatory Requirements Uk.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Medical Device Distributor Regulatory Requirements Uk Under those regulations, it’s possible, now,. You need to understand these requirements. The medical device regulations have significantly increased the requirements for distributors. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. The udi system provides a consistent and standard way to identify medical devices throughout their. Medical Device Distributor Regulatory Requirements Uk.
From pdfslide.net
(PDF) Medical Device and IVD Regulatory Conference Device and IVD Medical Device Distributor Regulatory Requirements Uk Under those regulations, it’s possible, now,. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. The medical device regulations have significantly increased the requirements for distributors. You need to understand these requirements. This guidance sets out what is required to place a medical device on. Medical Device Distributor Regulatory Requirements Uk.
From www.massoninternational.com
Mandatory Requirements a European Medical Device Distributor Must Meet Medical Device Distributor Regulatory Requirements Uk Under those regulations, it’s possible, now,. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. You need to understand these requirements. The medical. Medical Device Distributor Regulatory Requirements Uk.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Distributor Regulatory Requirements Uk While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. You need to understand these requirements. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. The udi system provides. Medical Device Distributor Regulatory Requirements Uk.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Distributor Regulatory Requirements Uk Under those regulations, it’s possible, now,. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. The udi system. Medical Device Distributor Regulatory Requirements Uk.
From www.ignitec.com
UK medical device regulations glossary What every medical... Medical Device Distributor Regulatory Requirements Uk The medical device regulations have significantly increased the requirements for distributors. You need to understand these requirements. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what. Medical Device Distributor Regulatory Requirements Uk.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Distributor Regulatory Requirements Uk The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. Under those regulations, it’s possible, now,. The medical device regulations have significantly increased. Medical Device Distributor Regulatory Requirements Uk.
From www.cell.com
The Regulation of Wearable Medical Devices Trends in Biotechnology Medical Device Distributor Regulatory Requirements Uk While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. Under those regulations, it’s possible, now,. The udi system provides. Medical Device Distributor Regulatory Requirements Uk.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I Medical Device Distributor Regulatory Requirements Uk The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. The medical device regulations have significantly increased the. Medical Device Distributor Regulatory Requirements Uk.
From www.mantrasystems.co.uk
Achieve EU MDR medical device compliance Free Guide 2024 Medical Device Distributor Regulatory Requirements Uk You need to understand these requirements. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. Under those regulations, it’s possible, now,. While previously it. Medical Device Distributor Regulatory Requirements Uk.
From www.hardianhealth.com
Medical Device Regulatory Services UKCA, CE and FDA — Hardian Health Medical Device Distributor Regulatory Requirements Uk The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. The medical device regulations have significantly increased the requirements for distributors. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. Under those regulations,. Medical Device Distributor Regulatory Requirements Uk.
From emmainternational.com
A Regulatory Strategy for your Medical Device EMMA International Medical Device Distributor Regulatory Requirements Uk Under those regulations, it’s possible, now,. The medical device regulations have significantly increased the requirements for distributors. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. This guidance sets out what is required to place a medical device on the gb,. Medical Device Distributor Regulatory Requirements Uk.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Medical Device Distributor Regulatory Requirements Uk This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. Under those regulations, it’s possible, now,. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. The medical device regulations have significantly increased. Medical Device Distributor Regulatory Requirements Uk.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Medical Device Distributor Regulatory Requirements Uk Under those regulations, it’s possible, now,. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. The medical device regulations have significantly increased the requirements for distributors. Check the legal requirements you need to meet before you can place a medical device on the market and see. Medical Device Distributor Regulatory Requirements Uk.
From admin.alacramed.com
UK medical device distributor legal manufacturer AlacraMed Medical Device Distributor Regulatory Requirements Uk The medical device regulations have significantly increased the requirements for distributors. Under those regulations, it’s possible, now,. You need to understand these requirements. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. This guidance sets out what is required to place a medical device on. Medical Device Distributor Regulatory Requirements Uk.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Medical Device Distributor Regulatory Requirements Uk The medical device regulations have significantly increased the requirements for distributors. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. The udi system provides. Medical Device Distributor Regulatory Requirements Uk.
From www.supplychain.nhs.uk
Extension to the Standstill Period for the UK Medical Devices Medical Device Distributor Regulatory Requirements Uk The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. Under those regulations, it’s possible, now,. The medical device regulations have significantly increased the requirements for distributors. While previously it was more common for an importer or distributor to also act as a ukrp, the newly. Medical Device Distributor Regulatory Requirements Uk.
From www.malvatronics.co.uk
Meeting Regulatory Requirements in Medical Device Software Development Medical Device Distributor Regulatory Requirements Uk The medical device regulations have significantly increased the requirements for distributors. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. Check the. Medical Device Distributor Regulatory Requirements Uk.
From www.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Device Distributor Regulatory Requirements Uk The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. You need to understand these requirements. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an in depth. Check the. Medical Device Distributor Regulatory Requirements Uk.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Medical Device Distributor Regulatory Requirements Uk The medical device regulations have significantly increased the requirements for distributors. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require an. Medical Device Distributor Regulatory Requirements Uk.
From www.spendmend.com
Medical Device Warranty Credits & Regulatory Requirements Medical Device Distributor Regulatory Requirements Uk The medical device regulations have significantly increased the requirements for distributors. The udi system provides a consistent and standard way to identify medical devices throughout their distribution and use by health care providers and patients. While previously it was more common for an importer or distributor to also act as a ukrp, the newly defined responsibilities of the ukrp require. Medical Device Distributor Regulatory Requirements Uk.
From familyclinic.netlify.app
Medical device regulations fda Medical Device Distributor Regulatory Requirements Uk Under those regulations, it’s possible, now,. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. Check the legal requirements you need to meet before you can place a medical device on the market and see how mhra. While previously it was more common for an importer. Medical Device Distributor Regulatory Requirements Uk.