What Is Boiling Point Graphite . In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite, mineral consisting of carbon. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. Graphite has a high melting point, similar to that of diamond. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. High melting and boiling points. Its properties are as follows: The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. It exhibits the properties of a metal and a nonmetal, which. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants.
from socratic.org
Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. Graphite has a high melting point, similar to that of diamond. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. It exhibits the properties of a metal and a nonmetal, which. High melting and boiling points. In order to melt graphite, it isn't enough to loosen one sheet from another. Its properties are as follows:
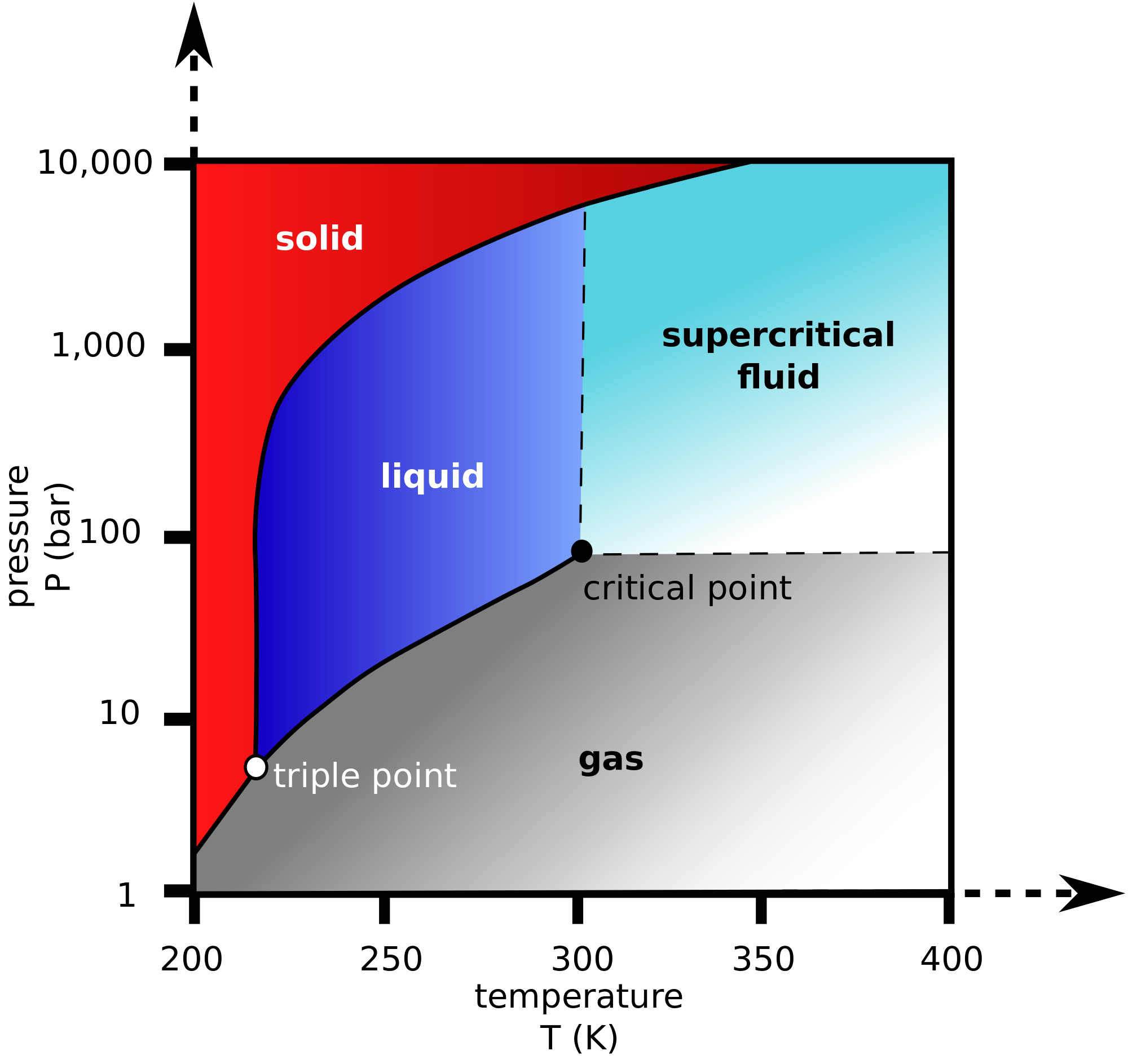
What is the relation between critical temperature and boiling point or vapor pressure? Socratic
What Is Boiling Point Graphite In order to melt graphite, it isn't enough to loosen one sheet from another. High melting and boiling points. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. Graphite has a high melting point, similar to that of diamond. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. In order to melt graphite, it isn't enough to loosen one sheet from another. It exhibits the properties of a metal and a nonmetal, which. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite, mineral consisting of carbon. Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Its properties are as follows:
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Is Boiling Point Graphite High melting and boiling points. Graphite, mineral consisting of carbon. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. It exhibits the properties of a metal and a nonmetal, which. The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Graphite. What Is Boiling Point Graphite.
From www.slideserve.com
PPT Chapter Introduction Lesson 1 Physical Properties Lesson 2 Density PowerPoint Presentation What Is Boiling Point Graphite It exhibits the properties of a metal and a nonmetal, which. In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite, mineral consisting of carbon. Graphite has a high melting point, similar to that of diamond.. What Is Boiling Point Graphite.
From themachine.science
The Boiling Point of Graphite A Comprehensive Exploration What Is Boiling Point Graphite Graphite, mineral consisting of carbon. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Graphite has a. What Is Boiling Point Graphite.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart What Is Boiling Point Graphite Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Its properties are as follows: Graphite, mineral consisting of carbon. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. It exhibits the properties of a metal and a nonmetal, which. In order to melt graphite, it. What Is Boiling Point Graphite.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is Boiling Point Graphite Graphite, mineral consisting of carbon. Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. It exhibits the properties of a metal and a nonmetal, which. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. Graphite has a greasy feel and leaves a black mark, thus the name from the. What Is Boiling Point Graphite.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is Boiling Point Graphite Its properties are as follows: Graphite, mineral consisting of carbon. High melting and boiling points. It exhibits the properties of a metal and a nonmetal, which. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. Graphite. What Is Boiling Point Graphite.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Is Boiling Point Graphite The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. Graphite, mineral consisting of carbon. Graphite has a high melting point, similar to that of diamond. High melting and boiling points. Graphite has a greasy. What Is Boiling Point Graphite.
From www.worksheetsplanet.com
What is Boiling Point What Is Boiling Point Graphite Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Graphite’s many covalent bonds are strong and substantial energy is. What Is Boiling Point Graphite.
From material-properties.org
Graphite Density, Strength, Hardness, Melting Point What Is Boiling Point Graphite High melting and boiling points. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. Graphite has a high melting point, similar to that of diamond. In order to melt graphite, it isn't enough. What Is Boiling Point Graphite.
From brainly.com
This graph shows the melting and boiling points of the alkali metals. Think about Francium, do What Is Boiling Point Graphite Graphite, mineral consisting of carbon. High melting and boiling points. Graphite has a high melting point, similar to that of diamond. It exhibits the properties of a metal and a nonmetal, which. In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite is a mineral that forms when carbon is subjected to heat and pressure. What Is Boiling Point Graphite.
From matmake.com
Boiling Point Definition, Measurements, and Applications What Is Boiling Point Graphite High melting and boiling points. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Its properties are as follows: Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. It exhibits the properties of a metal and a nonmetal, which. Graphite is an allotrope of carbon which is. What Is Boiling Point Graphite.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Is Boiling Point Graphite Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. High melting and boiling points. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. Graphite has a. What Is Boiling Point Graphite.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is Boiling Point Graphite It exhibits the properties of a metal and a nonmetal, which. In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power. What Is Boiling Point Graphite.
From internationalboilingpoint.weebly.com
Graphs International Boiling Point What Is Boiling Point Graphite Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. High melting and boiling points. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. It exhibits the properties of a metal and a nonmetal, which. Graphite has a high melting point, similar to that of. What Is Boiling Point Graphite.
From www.youtube.com
What is Boiling Point Concept of Boiling Point Boiling Point Formula Boiling Points YouTube What Is Boiling Point Graphite Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite’s many covalent bonds are strong and substantial energy is needed to. What Is Boiling Point Graphite.
From chem.libretexts.org
Fundamentals of Phase Transitions Chemistry LibreTexts What Is Boiling Point Graphite The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. It exhibits the properties of a metal and a nonmetal, which. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. Graphite is a mineral that forms when carbon is subjected to. What Is Boiling Point Graphite.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is Boiling Point Graphite The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. Graphite, mineral consisting of carbon. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants.. What Is Boiling Point Graphite.
From www.engineeringtoolbox.com
Hydrocarbons, alcohols and acids boiling points What Is Boiling Point Graphite Graphite, mineral consisting of carbon. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. It exhibits the properties of a metal and a nonmetal, which. Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. High melting and boiling points. Graphite has a high melting point, similar to that of diamond. Graphite is. What Is Boiling Point Graphite.
From www.nuclear-power.com
Carbon Melting Point Boiling Point What Is Boiling Point Graphite Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. High melting and boiling points. In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite, mineral consisting of carbon. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. Graphite’s many. What Is Boiling Point Graphite.
From www.studypool.com
SOLUTION Difference Between Diamond And Graphite Studypool What Is Boiling Point Graphite Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite occurs naturally in metamorphic. What Is Boiling Point Graphite.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is Boiling Point Graphite Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. High melting and boiling points. Its properties are as follows: The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. In order to melt graphite, it isn't enough to loosen one sheet. What Is Boiling Point Graphite.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID6431061 What Is Boiling Point Graphite In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. Graphite has a high melting point, similar to that of diamond. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. It exhibits the properties of a metal and a. What Is Boiling Point Graphite.
From www.saatchiart.com
BOILING GRAPHITE/ GRAPHITE EN ÉBULLITION Painting by Claude Lessard Saatchi Art What Is Boiling Point Graphite Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. Its properties are as follows: Graphite has a high melting point, similar to that of diamond. Graphite has a greasy feel and leaves a black mark, thus the name from the. What Is Boiling Point Graphite.
From favpng.com
Boiling Point Melting Point Heat Temperature Chemistry, PNG, 1538x1600px, Boiling Point, Boiling What Is Boiling Point Graphite High melting and boiling points. Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite is an allotrope of carbon which. What Is Boiling Point Graphite.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting boiling point of liquids What Is Boiling Point Graphite In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. High melting and boiling points. Graphite, mineral consisting of carbon. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. The purpose of. What Is Boiling Point Graphite.
From www.engineeringtoolbox.com
Boiling points of hydrocarbons, alcohols and acids What Is Boiling Point Graphite High melting and boiling points. The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite has a. What Is Boiling Point Graphite.
From www.wou.edu
CH105 Chapter 7 Alkanes and Halogenated Hydrocarbons Chemistry What Is Boiling Point Graphite In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. The purpose of this document is to introduce the reader. What Is Boiling Point Graphite.
From internationalboilingpoint.weebly.com
Graphs International Boiling Point What Is Boiling Point Graphite Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. Graphite, mineral consisting of carbon. In order to melt graphite,. What Is Boiling Point Graphite.
From socratic.org
What is the relation between critical temperature and boiling point or vapor pressure? Socratic What Is Boiling Point Graphite The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Graphite, mineral consisting of carbon. High melting and boiling points. Graphite has a high melting point, similar to that of diamond. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. Graphite. What Is Boiling Point Graphite.
From www.passmyexams.co.uk
Physical Properties of Alkanes Easy exam revision notes for GSCE Chemistry What Is Boiling Point Graphite Graphite has a high melting point, similar to that of diamond. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. In order to melt. What Is Boiling Point Graphite.
From www.jove.com
11369.jpg What Is Boiling Point Graphite High melting and boiling points. Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. Graphite is an allotrope of carbon which is used for making moderator rods in. What Is Boiling Point Graphite.
From www.slideshare.net
Chem matters ch7_covalent_bonding What Is Boiling Point Graphite Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. Its properties are as follows: Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. In order to melt graphite, it isn't enough to loosen one sheet from another. Graphite has a greasy feel and leaves a black mark, thus the. What Is Boiling Point Graphite.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols What Is Boiling Point Graphite High melting and boiling points. The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Graphite has a high melting point, similar to that of diamond. Its properties are as follows: It exhibits the properties of a metal and a nonmetal, which. Graphite is an allotrope of carbon which. What Is Boiling Point Graphite.
From www.youtube.com
Freezing and Boiling Point Graph YouTube What Is Boiling Point Graphite The purpose of this document is to introduce the reader to graphite properties and to describe testing techniques that enable true. Its properties are as follows: Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. It exhibits the properties of a metal and a nonmetal, which. Graphite is a mineral that forms when carbon is subjected to. What Is Boiling Point Graphite.
From wisc.pb.unizin.org
Melting and Boiling Point Comparisons (M10Q2) UWMadison Chemistry 103/104 Resource Book What Is Boiling Point Graphite Graphite is an allotrope of carbon which is used for making moderator rods in nuclear power plants. Graphite’s many covalent bonds are strong and substantial energy is needed to break them. Graphite is a mineral that forms when carbon is subjected to heat and pressure in earth's crust. Graphite has a high melting point, similar to that of diamond. Graphite. What Is Boiling Point Graphite.