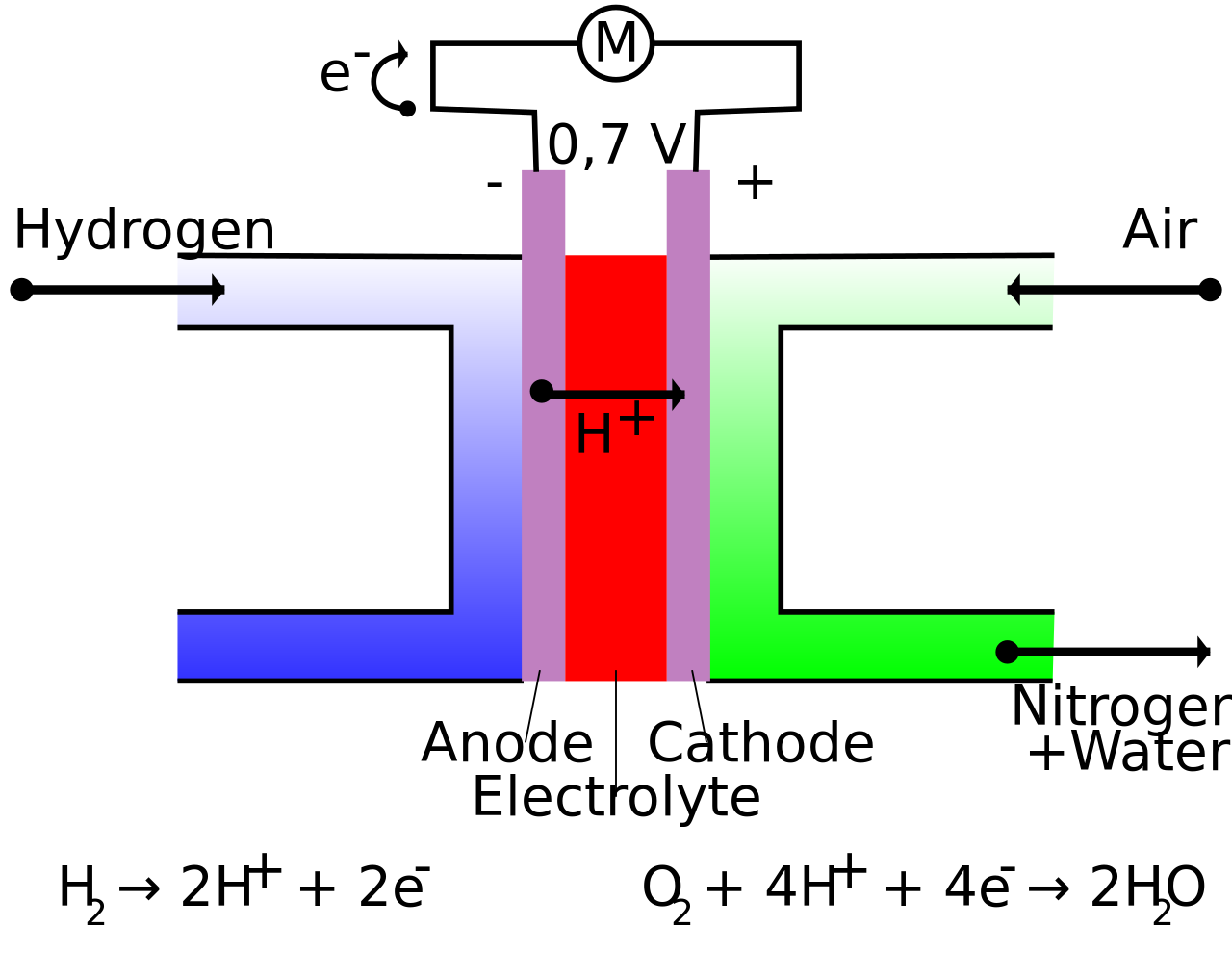
Fuel Cell And Hydrogen Equation . A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Hydrogen is oxidized to protons at the anode, and the electrons are. 2h2 + o2 → 2h2o. Hydrogen ions combine with 02. The overall equation for the reaction within a hydrogen fuel cell is: A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Into the cell and flows over the anode catalyst. Hydrogen + oxygen → water. Different electrolytes and fuels can be used to set up different types of fuel cells; The (chemical) energy content of any fuel is called heating value (for. A chemical cell produces a voltage until one of the reactants is used up.
from large.stanford.edu
A chemical cell produces a voltage until one of the reactants is used up. Different electrolytes and fuels can be used to set up different types of fuel cells; Hydrogen + oxygen → water. The (chemical) energy content of any fuel is called heating value (for. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Into the cell and flows over the anode catalyst. The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen is oxidized to protons at the anode, and the electrons are. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen ions combine with 02.
Hydrogen Fuel Cells
Fuel Cell And Hydrogen Equation Hydrogen is oxidized to protons at the anode, and the electrons are. Different electrolytes and fuels can be used to set up different types of fuel cells; The (chemical) energy content of any fuel is called heating value (for. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Into the cell and flows over the anode catalyst. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen ions combine with 02. A chemical cell produces a voltage until one of the reactants is used up. Hydrogen + oxygen → water. 2h2 + o2 → 2h2o. Hydrogen is oxidized to protons at the anode, and the electrons are.
From totalshield.com
Electrolyzer and Hydrogen Fuel Cell Safety TotalShield Fuel Cell And Hydrogen Equation The overall equation for the reaction within a hydrogen fuel cell is: Different electrolytes and fuels can be used to set up different types of fuel cells; A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A hydrogen fuel cell produces electrical energy directly from. Fuel Cell And Hydrogen Equation.
From www.researchgate.net
Block diagram of a hydrogenbased fuel cell. Download Scientific Diagram Fuel Cell And Hydrogen Equation The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen ions combine with 02. Different electrolytes and fuels can be used to set up different types of fuel cells; A chemical cell produces a voltage until one of the reactants is used up. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. A fuel. Fuel Cell And Hydrogen Equation.
From www.chegg.com
Solved Suppose you have a hydrogenoxygen fuel cell with the Fuel Cell And Hydrogen Equation Into the cell and flows over the anode catalyst. Different electrolytes and fuels can be used to set up different types of fuel cells; Hydrogen ions combine with 02. A chemical cell produces a voltage until one of the reactants is used up. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The (chemical) energy content of. Fuel Cell And Hydrogen Equation.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Cell And Hydrogen Equation Different electrolytes and fuels can be used to set up different types of fuel cells; Hydrogen + oxygen → water. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The (chemical) energy content of any fuel is called heating value (for. Hydrogen is oxidized to protons at the anode, and the electrons are. A fuel cell like. Fuel Cell And Hydrogen Equation.
From www.instructables.com
How to Make a Simple Hydrogen Fuel Cell 8 Steps Instructables Fuel Cell And Hydrogen Equation The (chemical) energy content of any fuel is called heating value (for. Hydrogen ions combine with 02. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The overall equation for the reaction within a hydrogen fuel cell is: A chemical cell produces a voltage until one of the reactants is used up. Different electrolytes and fuels can. Fuel Cell And Hydrogen Equation.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cell And Hydrogen Equation Hydrogen is oxidized to protons at the anode, and the electrons are. 2h2 + o2 → 2h2o. The overall equation for the reaction within a hydrogen fuel cell is: The (chemical) energy content of any fuel is called heating value (for. Hydrogen ions combine with 02. A chemical cell produces a voltage until one of the reactants is used up.. Fuel Cell And Hydrogen Equation.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell Fuel Cell And Hydrogen Equation A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen is oxidized to protons at the anode, and the electrons are. Into the cell and flows over the anode catalyst. 2h2 + o2 → 2h2o. The (chemical) energy content of any fuel is called heating. Fuel Cell And Hydrogen Equation.
From www.slideserve.com
PPT Fuel Cell Catalysts Based on Metal Nanoparticles PowerPoint Fuel Cell And Hydrogen Equation 2h2 + o2 → 2h2o. Different electrolytes and fuels can be used to set up different types of fuel cells; Hydrogen is oxidized to protons at the anode, and the electrons are. A chemical cell produces a voltage until one of the reactants is used up. Into the cell and flows over the anode catalyst. Hydrogen + oxygen → water.. Fuel Cell And Hydrogen Equation.
From doren-project.medium.com
Relation Between Fuel Cells and Hydrogen DoRen Medium Fuel Cell And Hydrogen Equation Different electrolytes and fuels can be used to set up different types of fuel cells; Hydrogen is oxidized to protons at the anode, and the electrons are. 2h2 + o2 → 2h2o. The (chemical) energy content of any fuel is called heating value (for. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. A fuel cell like. Fuel Cell And Hydrogen Equation.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cell And Hydrogen Equation 2h2 + o2 → 2h2o. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen ions combine with 02. A chemical cell produces a voltage until one of the reactants is used up. Into the cell and flows over the anode catalyst. The (chemical) energy content of any fuel is called heating value (for. The overall equation. Fuel Cell And Hydrogen Equation.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell Fuel Cell And Hydrogen Equation The (chemical) energy content of any fuel is called heating value (for. Different electrolytes and fuels can be used to set up different types of fuel cells; 2h2 + o2 → 2h2o. The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen ions combine with 02. A fuel cell like this will continue to operate and produce. Fuel Cell And Hydrogen Equation.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube Fuel Cell And Hydrogen Equation The (chemical) energy content of any fuel is called heating value (for. Hydrogen ions combine with 02. Hydrogen is oxidized to protons at the anode, and the electrons are. 2h2 + o2 → 2h2o. Into the cell and flows over the anode catalyst. Hydrogen + oxygen → water. A fuel cell like this will continue to operate and produce electrical. Fuel Cell And Hydrogen Equation.
From www.youtube.com
Advantages and disadvantages of hydrogen fuel cell engines YouTube Fuel Cell And Hydrogen Equation A chemical cell produces a voltage until one of the reactants is used up. Hydrogen + oxygen → water. Different electrolytes and fuels can be used to set up different types of fuel cells; The (chemical) energy content of any fuel is called heating value (for. The overall equation for the reaction within a hydrogen fuel cell is: 2h2 +. Fuel Cell And Hydrogen Equation.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cell And Hydrogen Equation Into the cell and flows over the anode catalyst. The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen ions combine with 02. A chemical cell produces a voltage until one of the reactants is used up. Different electrolytes and fuels can be used to set up different types of fuel cells; Hydrogen + oxygen → water.. Fuel Cell And Hydrogen Equation.
From www.slideshare.net
Hydrogen fuel cells Fuel Cell And Hydrogen Equation 2h2 + o2 → 2h2o. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The (chemical) energy content of any fuel is called heating value (for. Hydrogen is oxidized to protons at the anode, and the electrons are. Hydrogen + oxygen → water. Hydrogen ions combine with 02. Different electrolytes and fuels can be used to set. Fuel Cell And Hydrogen Equation.
From www.researchgate.net
Schematic diagram of hydrogen fuel cell Download Scientific Diagram Fuel Cell And Hydrogen Equation The overall equation for the reaction within a hydrogen fuel cell is: A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Into the cell and flows over the anode catalyst. Different electrolytes and fuels can be used to set up different types of fuel cells;. Fuel Cell And Hydrogen Equation.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Fuel Cell And Hydrogen Equation Hydrogen + oxygen → water. A chemical cell produces a voltage until one of the reactants is used up. The overall equation for the reaction within a hydrogen fuel cell is: Into the cell and flows over the anode catalyst. 2h2 + o2 → 2h2o. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen ions combine. Fuel Cell And Hydrogen Equation.
From www.youtube.com
How does a hydrogen fuel cell work? (AKIO TV) YouTube Fuel Cell And Hydrogen Equation A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The (chemical) energy content of any fuel is called heating value (for. Hydrogen + oxygen → water. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Into the cell and flows over the. Fuel Cell And Hydrogen Equation.
From www.researchgate.net
Schematic illustration of a hydrogen fuel cell. Download Scientific Fuel Cell And Hydrogen Equation A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen + oxygen → water. Hydrogen ions combine with 02. The (chemical) energy content of any fuel is called heating value (for. The overall equation for the reaction within a hydrogen fuel cell is: Into the cell and flows over the anode catalyst. Hydrogen is oxidized to protons. Fuel Cell And Hydrogen Equation.
From chem.libretexts.org
2.2. Hydrogen, The Simplest Atom Chemistry LibreTexts Fuel Cell And Hydrogen Equation A hydrogen fuel cell produces electrical energy directly from a chemical reaction. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A chemical cell produces a voltage until one of the reactants is used up. 2h2 + o2 → 2h2o. Hydrogen + oxygen → water.. Fuel Cell And Hydrogen Equation.
From www.youtube.com
Trick for H2 O2 Fuel cells YouTube Fuel Cell And Hydrogen Equation Into the cell and flows over the anode catalyst. 2h2 + o2 → 2h2o. Hydrogen ions combine with 02. Hydrogen is oxidized to protons at the anode, and the electrons are. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The overall equation for the reaction within a hydrogen fuel cell is: The (chemical) energy content of. Fuel Cell And Hydrogen Equation.
From www.researchgate.net
6. Hydrogenoxygen fuel cell scheme. Download Scientific Diagram Fuel Cell And Hydrogen Equation A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The (chemical) energy content of any fuel is called heating value (for. Different electrolytes and fuels can be used to set up different types of fuel cells; Into the cell and flows over the anode catalyst. Hydrogen ions combine with 02. A chemical cell produces a voltage until. Fuel Cell And Hydrogen Equation.
From www.nagwa.com
Question Video Combining Equations to Give the Overall Reaction for a Fuel Cell And Hydrogen Equation The (chemical) energy content of any fuel is called heating value (for. The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen + oxygen → water. A chemical cell produces a voltage until one of the reactants is used up. Into the cell and flows over the anode catalyst. Different electrolytes and fuels can be used to. Fuel Cell And Hydrogen Equation.
From www.linquip.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip Fuel Cell And Hydrogen Equation Into the cell and flows over the anode catalyst. The (chemical) energy content of any fuel is called heating value (for. Hydrogen + oxygen → water. A chemical cell produces a voltage until one of the reactants is used up. 2h2 + o2 → 2h2o. The overall equation for the reaction within a hydrogen fuel cell is: Different electrolytes and. Fuel Cell And Hydrogen Equation.
From www.cummins.com
Five key questions about the next frontier Hydrogen fuel cells Fuel Cell And Hydrogen Equation 2h2 + o2 → 2h2o. Hydrogen ions combine with 02. The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen + oxygen → water. Different electrolytes and fuels can be used to set up different types of fuel cells; Into the cell and flows over the anode catalyst. A hydrogen fuel cell produces electrical energy directly from. Fuel Cell And Hydrogen Equation.
From energytracker.asia
The Pros and Cons of Hydrogen Fuel Cells Fuel Cell And Hydrogen Equation Different electrolytes and fuels can be used to set up different types of fuel cells; 2h2 + o2 → 2h2o. Hydrogen + oxygen → water. The (chemical) energy content of any fuel is called heating value (for. The overall equation for the reaction within a hydrogen fuel cell is: A chemical cell produces a voltage until one of the reactants. Fuel Cell And Hydrogen Equation.
From www.researchgate.net
Oxidation reactions in fuel cells (cathodic and anodic half reactions Fuel Cell And Hydrogen Equation Hydrogen + oxygen → water. Different electrolytes and fuels can be used to set up different types of fuel cells; Hydrogen ions combine with 02. The overall equation for the reaction within a hydrogen fuel cell is: A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The (chemical) energy content of any fuel is called heating value. Fuel Cell And Hydrogen Equation.
From mavink.com
Hydrogen Fuel Cell Design Fuel Cell And Hydrogen Equation Hydrogen ions combine with 02. The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen is oxidized to protons at the anode, and the electrons are. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Different electrolytes and fuels can be used. Fuel Cell And Hydrogen Equation.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Cell And Hydrogen Equation The (chemical) energy content of any fuel is called heating value (for. 2h2 + o2 → 2h2o. The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen + oxygen → water. A chemical cell produces a voltage until one of the reactants is used up. A fuel cell like this will continue to operate and produce electrical. Fuel Cell And Hydrogen Equation.
From www.air101.co.uk
Air101 Hydrogen fuel cells, explained Fuel Cell And Hydrogen Equation Hydrogen ions combine with 02. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The overall equation for the reaction within a hydrogen fuel cell is: 2h2 + o2 → 2h2o. Different electrolytes and fuels can be used to set up different types of fuel. Fuel Cell And Hydrogen Equation.
From www.designlife-cycle.com
Hydrogen Fuel Cell — Design LifeCycle Fuel Cell And Hydrogen Equation The overall equation for the reaction within a hydrogen fuel cell is: A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen is oxidized to protons at the anode, and the electrons are. 2h2 + o2 → 2h2o. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of. Fuel Cell And Hydrogen Equation.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Fuel Cell And Hydrogen Equation Different electrolytes and fuels can be used to set up different types of fuel cells; The overall equation for the reaction within a hydrogen fuel cell is: The (chemical) energy content of any fuel is called heating value (for. Hydrogen is oxidized to protons at the anode, and the electrons are. A chemical cell produces a voltage until one of. Fuel Cell And Hydrogen Equation.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Fuel Cell And Hydrogen Equation A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen is oxidized to protons at the anode, and the electrons are. 2h2 + o2 → 2h2o. Into the cell and flows over the anode catalyst. Hydrogen + oxygen → water. The (chemical) energy content of any fuel is called heating value (for. Hydrogen ions combine with 02.. Fuel Cell And Hydrogen Equation.
From www.savemyexams.com
Fuel Cells SL IB Chemistry Revision Notes 2025 Fuel Cell And Hydrogen Equation Hydrogen ions combine with 02. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. The (chemical) energy content of any fuel is called heating value (for. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The overall equation for the reaction within. Fuel Cell And Hydrogen Equation.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Fuel Cell And Hydrogen Equation The overall equation for the reaction within a hydrogen fuel cell is: Hydrogen is oxidized to protons at the anode, and the electrons are. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The (chemical) energy content of any fuel is called heating value (for.. Fuel Cell And Hydrogen Equation.